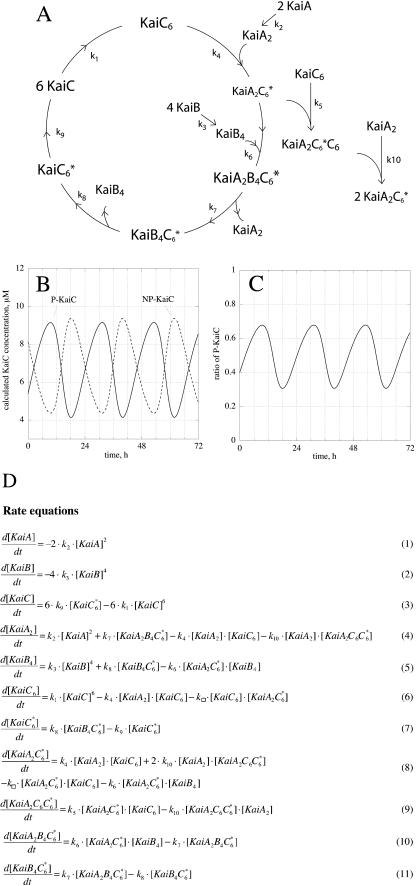
Figure 6. An Expanded Model of the KaiC Oscillator Containing Explicit Stoichiometries.
(A) KaiXkYlZm denotes a complex between k KaiX, l KaiY, and m KaiZ molecules.
(B) Calculated oscillations of total phosphorylated KaiC (P-KaiC) and total unphosphorylated KaiC (NP-KaiC).
(C) Calculated oscillatory ratio of P-KaiC = P-KaiC/(P-KaiC + NP-KaiC).
(D) Rate equations of the model. The following rate constants and initial concentrations were used in (B) and (C): k1 = 0.1 μM−5 h−1, k2 = 0.1 μM−1 h−1, k3 = 0.1 μM−3 h−1, k4 = 0.001 μM−1 h−1, k5 = 1.8 μM−1 h−1, k6 = 2.5 μM−1 h−1, k7 = 1.0 h−1, k8 = 1.0 h−1, k9 = 0.1 h−1, and k10 = 40 μM−1 h−1. [KaiA] = 0.0161 μM, [KaiA2 B4C*6] = 0.0611 μM, [KaiA2C*6] = 0.0205 μM, [KaiA2C*6C6] = 7.4 × 10−4 μM, [KaiA2] = 0.9094 μM, [KaiB] = 0.1402 μM, [KaiB4C*6] = 0.0894 μM, [KaiB4] = 0.7395 μM, [KaiC] = 1.0288 μM, [KaiC*6] = 1.1805 μM, and [KaiC6] = 0.7256 μM.