FIGURE 1. Nonhydrolyzed ATP can activate the in vitro generation of Aβ40 and Aβ42.
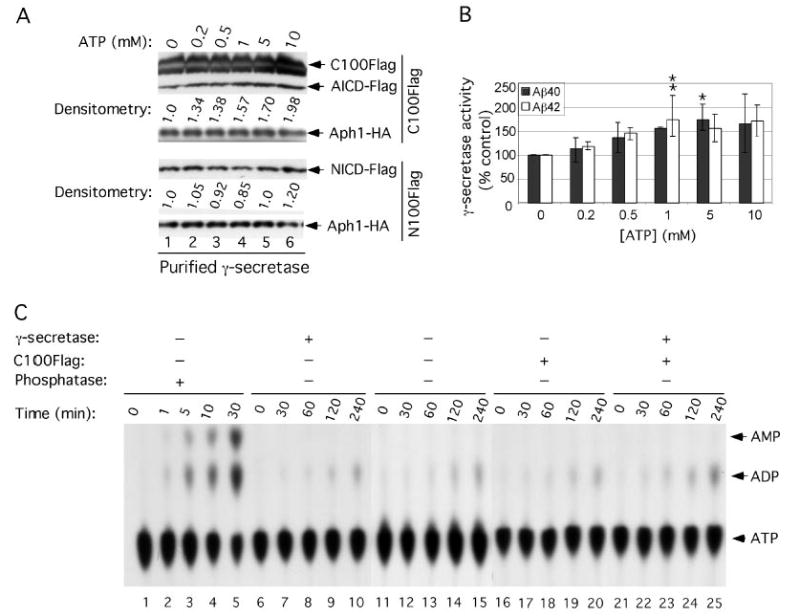
A, effect of ATP on purified γ-secretase activity. γ-Secretase diluted in 0.2% CHAPSO/HEPES, pH 7.5, was incubated at 37 °C for 4 h in the presence of 0.1% PC, 0.025% PE, the indicated concentrations of ATP, and 1 μm C100FLAG (an APP-based substrate) or 1 μm N100FLAG (a Notch-based substrate); both substrates were adjusted to 0.5% SDS prior to addition to the reactions (6). The reactions were Western-blotted for AICD-FLAG (with M2 anti-FLAG antibody) and for NICD-FLAG (with Notch Ab1744 antibody). Levels of Aph1-HA serve as equal loading controls. Aβ40 and Aβ42 were measured by ELISA (B, means ± S.D.; n = 3). Levels of AICD-FLAG and NICD-FLAG were estimated by densitometry (values are single determinations from the blot shown). Asterisks indicate significant differences in Aβ40 (*, p < 0.01) and Aβ42 (**, p < 0.01) production compared with samples without ATP. C, ATPase assays on purified γ-secretase. [α-32P]ATP was incubated at 37 °C for the indicated times in the reaction buffer (0.2% CHAPSO/HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 5 mm CaCl2, 0.025% PE, and 0.10% PC) alone (lanes 11–15) or in the presence of purified γ-secretase (lanes 6 –10), C100FLAG substrate (lanes 16 –20), or both purified γ-secretase and C100FLAG substrate (lanes 21–25). Two μl of each reaction were then analyzed by TLC to separate ATP from ADP. As a positive control to show ATP hydrolysis products, [α-32P]ATP was incubated at the indicated times in the presence of canine kidney phosphatase (lanes 1–5).
