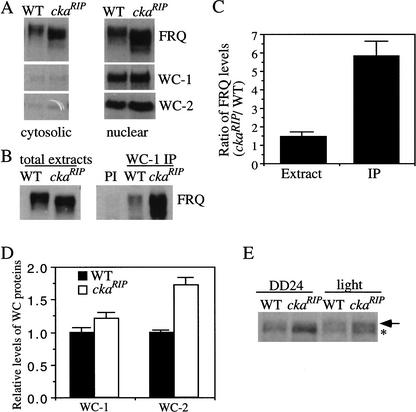
Figure 6.
The nuclear localization of FRQ was normal in the ckaRIP mutant, and significantly more of the hypophosphorylated FRQ in the ckaRIP strain were found to be associated with the WC proteins than FRQ in the wild-type strain. (A) Western blot analysis showing that FRQ in the ckaRIP mutant was found in the nucleus. Western blots were probed with FRQ, WC-1, and WC-2 antiserum. (B) Immunoprecipitation assay showing that more of the FRQ protein in the ckaRIP strain was found to coprecipitate with the WC proteins than the wild type. The Neurospora protein extracts (LL) were either immunoprecipitated by WC-1 antiserum (right) or analyzed directly (left) by Western blot analyses. (PI) Wild-type extracts were immunoprecipitated by WC-1 pre-immune serum. Note the similar FRQ phosphorylation pattern in wild-type and the ckaRIP strain after immunoprecipitation. (C) Densitometric analyses of the results from four independent immunoprecipitation experiments as described in B. Error bars, SD. (D) Comparison of the WC levels in the wild-type and the ckaRIP strains. The data is quantified from five independent experiments for cultures harvested in constant darkness (DD24 or DD28). (▪) Wild type; (□) ckaRIP. (E) Western blot analysis showing the comparison of the phosphorylation patterns of WC-1 in wild-type and ckaRIP strains. The light samples were harvested after a 15-min light pulse at DD24. To resolve the phosphorylated WC-1 bands, the electrophoresis time was twice as long as usual. The arrow indicates the hyperphosphorylated WC-1 band, whereas the asterisk marks the hypophosphorylated WC-1 band.