Figure 3.
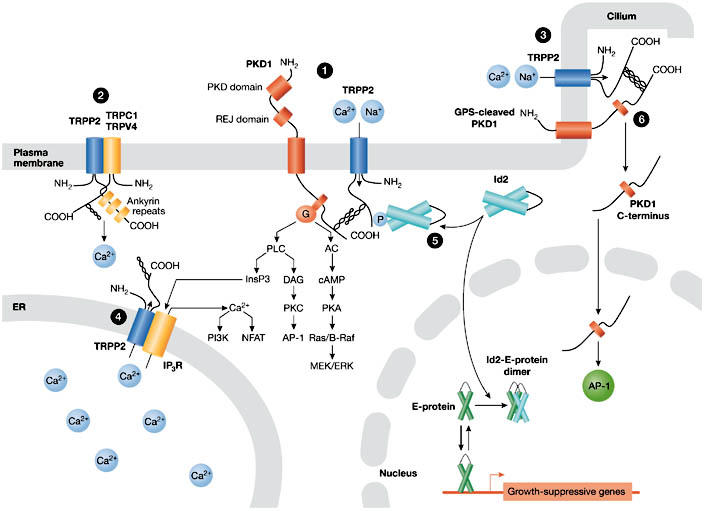
Compartment-specific functions of transient receptor potential polycystin 2. The various models for the localization-dependent functions of TRPP2 are shown. TRPP2 mediates Ca2+ influx at the plasma membrane (PM) and ciliary membrane (cilium), where it functions in a protein complex with polycystin 1 (PKD1) (1, 3) and possibly with other members of the TRP channel superfamily (TRPC1, TRPV4) (2). Ca2+ influx induced by shear flow in renal epithelial cells is triggered by bending of the luminal cilium and seemingly requires the presence of PKD1–TRPP2 complexes in the cilium (3). Mechanical stress results in activation of PKD1–TRPP2 complexes to allow Ca2+ influx either in the shaft or in the base of the cilium. TRPP2 acts as a Ca2+-release channel in the endoplasmic reticulum (ER), where it might interact with, and regulate, inositol-1,4,5-trisphosphate receptors (Ins(1,4,5)P3Rs) (4). Serine-phosphorylated TRPP2 sequesters Id2 in the cytoplasm and prevents it from entering the nucleus (5). PKD1 can undergo a proteolytic cleavage that releases its carboxy-terminal tail, which translocates to the nucleus and activates the transcription factor AP1 (6). Note that it is hypothesized that PKD1 targeted to the primary cilium is also cleaved at its GPCR proteolytic site (GPS). ERK, extracellular signal-regulated kinase; GPCR, G-protein coupled receptor; Id2, inhibitor of DNA binding 2; MEK, mitogen-activated protein kinase/ERK kinase; NFAT, nuclear factor of activated T-cells; PI3K, phosphatidyl-inositol 3-kinase; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; REJ, receptor for egg jelly; TRPP2, transient receptor potential polycystin 2.
