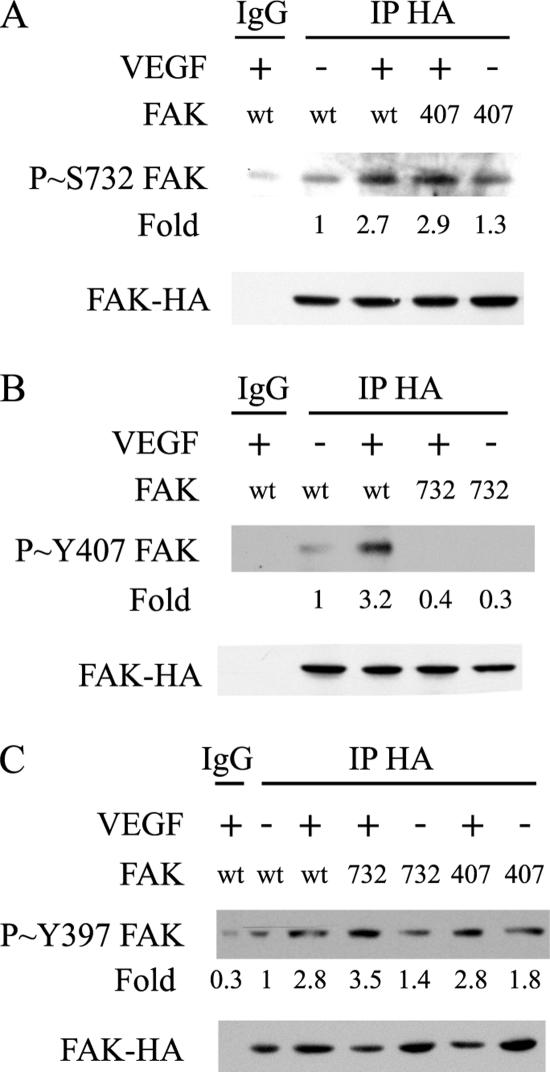
Figure 3.
VEGF-induced phosphorylation of FAK on Tyr407, but not on Tyr397, requires the phosphorylation of FAK on Ser732. (A) Quiescent HUVECs transiently expressing wild-type HA-tagged FAK (wt) or HA-tagged FAK mutant Tyr407Phe (407) were treated or not with 5 ng/ml VEGF for 10 min. After extraction, the HA-tagged proteins were immunoprecipitated using anti-HA mouse antibody. Control immunoprecipitation was done similarly using purified mouse IgG. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-FAK Ser732 (top) and total FAK-HA (bottom). Representative blots of three separate experiments are shown. (B) Quiescent HUVEC transiently expressing HA-tagged FAK (wt) or HA-tagged FAK-Ser732Ala (732) were treated or not with 5 ng/ml VEGF for 10 min. Cell extracts were prepared as described in A, and the membrane was processed for immunodetection of phospho-FAK Tyr407 (top) and total FAK-HA (bottom). Representative blots of two separate experiments are shown. (C) Quiescent HUVECs transiently expressing wild-type HA-tagged FAK (wt), HA-tagged FAK mutants Ser732Ala (732), or Tyr407Phe (407) were treated or not with 5 ng/ml VEGF for 10 min. After extraction, the HA-tagged proteins were immunoprecipitated using anti-HA mouse antibody and processed as described in A. The membrane was processed for immunodetection of phospho-FAK Tyr397 (top) and total FAK-HA (bottom).