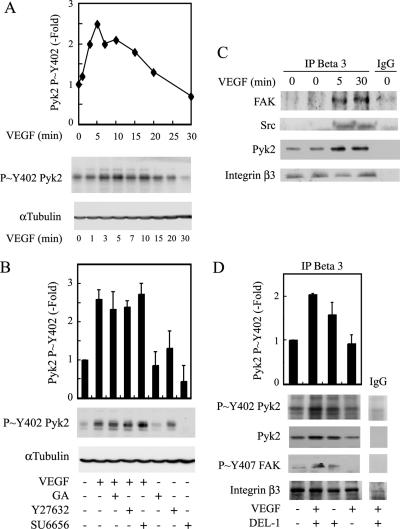
Figure 6.
Pyk2 is phosphorylated in an HSP90- and ROCK-independent manner in response to VEGF, and it associates with integrin β3 subunit. (A) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for increasing periods. Cells were then extracted, and proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Pyk2 Tyr402 (top), and total α-tubulin (bottom) to monitor loading. Representative autoradiograms are shown, and data points represent means of two separate experiments. (B) Quiescent HUVECs were pretreated for 60 min with 1 μg/ml geldanamycin (GA) or vehicle (0.25% DMSO), or for 120 min with 25 μM Y27632 or 5 μM SU6656. Cells were treated or not with 5 ng/ml VEGF for 5 min and were extracted and processed as described in A. Representative blots are shown, and data points represent means ± SD of three experiments. (C) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for 5 and 30 min. Cells were extracted, and integrin β3 was immunoprecipitated. Control immunoprecipitation was done similarly using preimmune mouse IgG. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for Western blot using anti-FAK mouse antibody, anti-Src mouse antibody, and anti-Pyk2 mouse antibody. The membrane was reprobed for total integrin β3 using anti-β3 mouse antibody to ensure equal protein loading (fourth panel). (D) HUVECs were trypsinized and left in suspension for 20 min in serum-free medium. Then, peptide 75 nM DEL-1 was added or not for 30 min. Suspended cells were treated or not with 5 ng/ml VEGF for the last 5 min. Cells were then extracted, and proteins were separated on SDS-PAGE and transferred on nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Pyk2 Tyr402, total Pyk2, and phospho-FAK Tyr407. The membrane was reprobed for total integrin β3 to ensure equal protein loading (fourth panel). Representative blots are shown, and data points represent means ± SD of duplicate sample of two experiments.