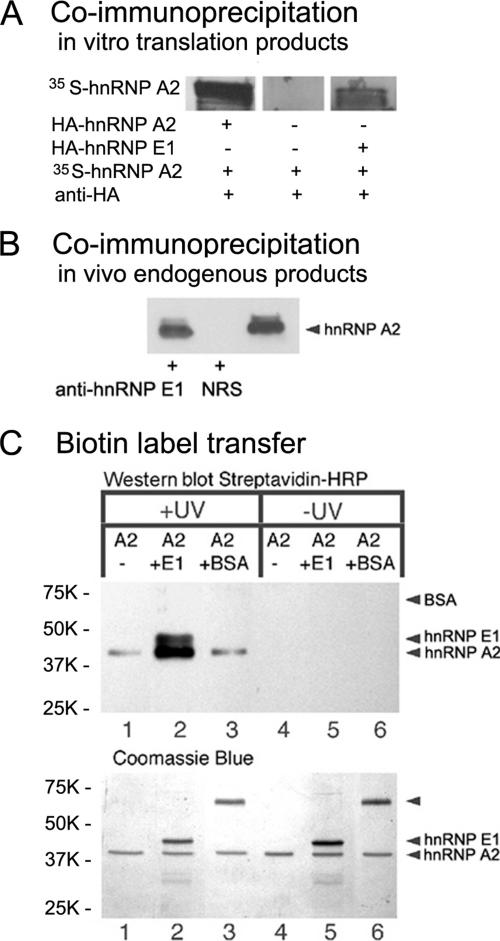
Figure 1.
hnRNP E1–hnRNP A2 interaction. (A) Coimmunoprecipitation: in vitro translation products. In vitro synthesized [35S]methionine-labeled hnRNP A2 was combined with a fivefold molar excess of hnRNP A2-HA or hnRNP E1-25-HA, and the mixture was precipitated with anti-HA antibodies. The precipitates were separated by SDS-PAGE; the gel was fixed, dried, and exposed to x-ray film. (B) Coimmunoprecipitation: in vivo endogenous products. Oligodendrocyte homogenate was incubated with rabbit anti-hnRNP E1 or with normal rabbit serum (NRS). The immunoprecipitates were isolated with protein A-conjugated agarose beads, separated by SDS-PAGE, and subjected to Western blot analysis for the presence of hnRNP A2 using mouse anti-hnRNP A2. The right lane is an aliquot of the homogenate before immunoprecipitation. (C) Biotin transfer assay: hnRNP A2 (A2) conjugated with Sulfo-SBED was mixed with hnRNP E1 (E1), with BSA, or without any addition, and subjected (+) or not (−) to UV cross-linking. After reduction, the protein mixtures were analyzed by SDS-PAGE, blotted onto PVDF membrane, and probed with streptavidin-horseradish peroxidase (top). Corresponding portions were analyzed by SDS-PAGE and stained with Coomassie blue (bottom). Molecular weight markers are displayed to the left.