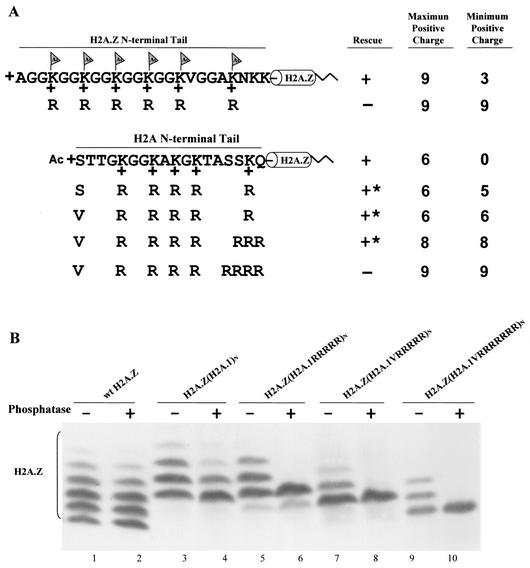
FIG. 4.
The major H2A N-terminal tail can replace the essential function of the H2A.Z variant N-terminal tail. (A) Constructs in which the N-terminal tail of H2A.Z, whose acetylation has an essential function, was replaced either by a wild-type H2A.1 tail or by mutated tails containing increasing numbers of arginine replacements were transformed into H2A.Z knockout heterokaryons. Transformants with the chimeric gene containing a wild-type H2A.1 N-terminal tail on H2A.Z grow normally. Tetrahymena cells can also survive with arginine replacement mutations which increase the total positive charge of the N-terminal tail to +8 but cannot survive when the total positive charge is increased to +9. ∗, mutants with severe phenotypes, including slow growth, variable size, and irregular surfaces. (B) Macronuclear histones from viable transformants were extracted, blotted, and detected with a specific antibody to H2A.Z. The banding patterns of the chimeric proteins (H2A.1 tails on H2A.Z) caused by acetylation are similar to those of the major H2A.1, with three or four phosphatase-resistant isoforms for H2A.Z(H2A.1)N and two for H2A.Z(H2A.1RRRRR)N. H2A.Z(H2A.1VRRRRR)N and H2A.Z(H2A.1VRRRRRRR)N both show a single phosphatase-resistant isoform.