FIG. 5.
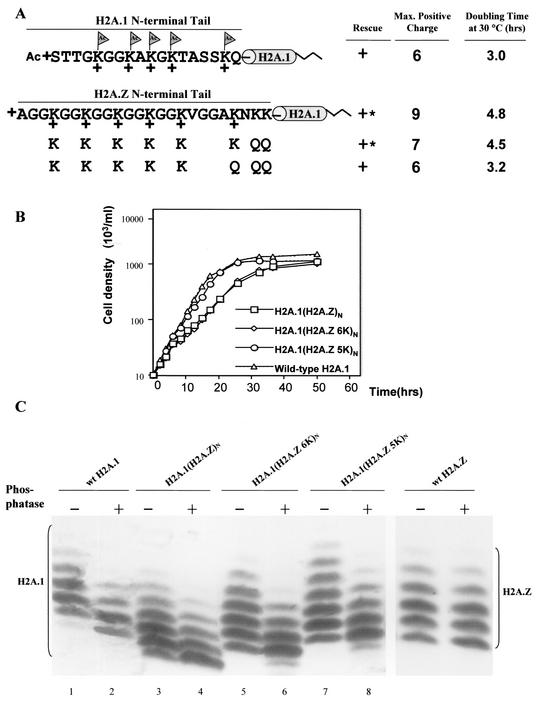
The H2A.Z N-terminal tail can also replace the function of the major H2A N-terminal tail. (A) The N-terminal tail of H2A.1 was replaced either by the wild-type H2A.Z N-terminal tail or by tails with different numbers of glutamine replacements at nonacetylatable residues that reduce the positive charges of the N-terminal tail without eliminating acetylation sites. The wild-type H2A.Z N-terminal tail on H2A.1 yields transformants with a slow-growth phenotype, while glutamine replacement mutations, which decrease the maximum positive charge of the chimeric protein's N-terminal tail to +6, generate transformants whose doubling time at 30°C is indistinguishable from that of wild-type cells. ∗, mutants with severe phenotypes, including slow growth, variable size, and irregular surfaces. (B) Mutant H2A.1(H2A.Z)N, H2A.1(H2A.Z 6K)N, and H2A.1(H2A.Z 5K)N, as well as strains rescued with the wild-type gene, were grown in 1× SPP medium at 30°C. Cell densities were measured for up to 50 h and plotted on a log scale. Doubling times in hours are listed in Fig. 5A. (C) Macronuclear histones from the mutants were extracted, blotted, and detected with a specific antibody to major H2A. While wild-type H2A.1 has four or five phosphatase-resistant isoforms, the chimeric protein, H2A.1(H2A.Z)N, shows five or six phosphatase-resistant isoforms, a pattern similar to that of wild-type H2A.Z. H2A.1(H2A.Z 6K)N and H2A.1(H2A.Z 5K)N show patterns similar to that of H2A.1(H2A.Z)N, except for small mobility differences likely caused by the extra glutamine mutations that abolish two or three positive charges on the N-terminal tail.