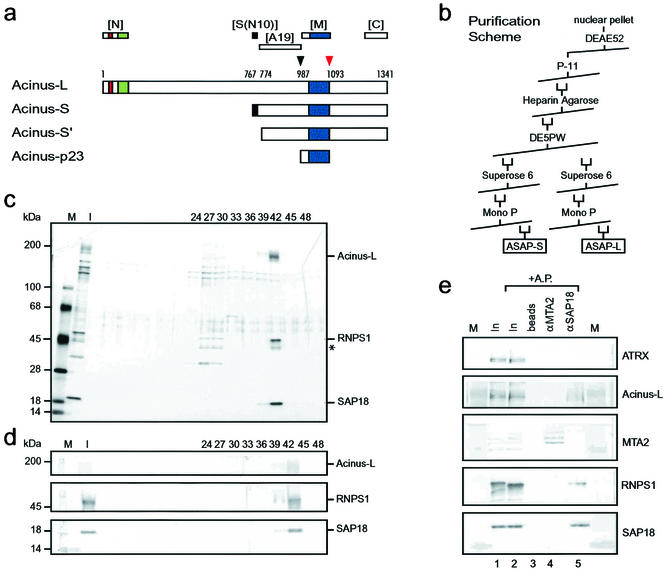
FIG. 1.
Purification of a complex consisting of SAP18, RNPS1, and Acinus-L. (a) Schematic representation of Acinus isoforms. Described protein domains as well as protease cleavage sites and their corresponding amino acids (1, 30) are indicated as follows: red box, P-loop for nucleotide binding; green box, SAP domain; dark blue box, region similar to RNA recognition motif of Drosophila Sxl; black box, region specific for Acinus-S; black arrowhead, cleavage site for unknown protease; red arrowhead, cleavage site for caspase 3. Regions recognized by polyclonal antibodies against Acinus ([N], [S(N10)], [A19], [M], and [C]) (see also Materials and Methods) are shown above the representation of Acinus-L. (b) Purification scheme used to isolate the ASAP complexes from HeLa cell nuclear pellets. (c) Silver stain of an SDS-4 to 15% (wt/vol) polyacrylamide gradient gel containing aliquots of fractions (input [I] and the indicated column fractions) derived from the MonoP column. High-molecular-mass standards [M] are indicated at the left, and components of the ASAP complex are indicated at the right side of the panel. A putative alternatively phosphorylated or partially degraded form of RNPS1 is marked with an asterisk. (d) Western blot analysis of the samples used in panel c. The antibodies used in the Western blot are indicated at the right, and high-molecular-mass standards [M] are indicated at the left side of the panel. (e) Verification of the complex composition by coimmunoprecipitation. Antibodies against SAP18 (lane 5) but not MTA-2 (lane 4) coimmunoprecipitate RNPS1 and Acinus-L from a protein fraction derived from nuclear pellet [In]. Samples in lanes 2 to 5 were treated with alkaline phosphatase [AP] after immunoprecipitation in an attempt to increase the signal for Acinus-L by focusing putative differently phosphorylated species. Ten percent of the input is loaded in lanes 1 and 2. Immunoprecipitated proteins were separated on an SDS-4 to 15% (wt/vol) polyacrylamide gradient gel and analyzed by Western blotting with the antibodies indicated at the right. Lanes labeled [M] contain high-molecular-mass protein standards.