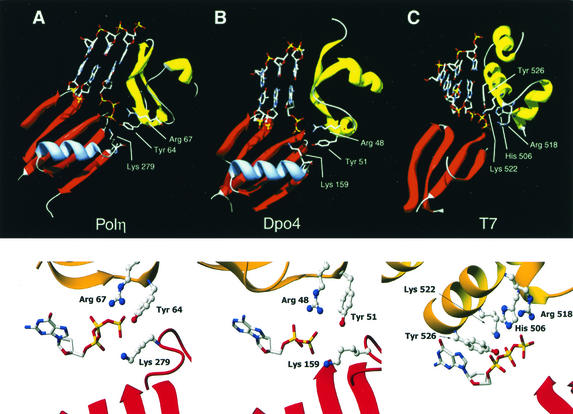
FIG. 2.
Contacts between the conserved residues and the incoming nucleotide. (A) Overview (top) and close-up view (bottom) of the positions of Tyr 64, Arg 67, and Lys 279 of yeast Polη in relation to the incoming deoxynucleotide, as predicted from the crystal structure. Tyr 64 and Arg 67 lie in the finger domain, and Lys 279 lies in a helix-turn above the palm domain. The DNA and nucleotide were modeled into the Polη structure by superposition of the Polη palm domain onto the equivalent domain in the T7 Pol/template-primer/ddGTP ternary complex. (B) Overview (top) and close-up view (bottom) of the homologous conserved Tyr, Arg, and Lys residues in the crystal structure of S. solfataricus Dpo4 protein with DNA and incoming dNTP. In this structure, the incoming ddATP was hydrolyzed to ddADP (12). (C) Overview (top) and close-up view (bottom) of amino acid residues in the T7 Pol/template-primer/ddGTP crystal structure that contact the incoming dNTP. In all cases, only the relevant regions of the fingers (yellow/gold) and palm (red) domains are shown.