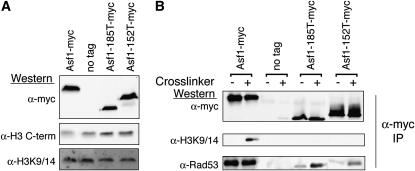
Figure 6.
The Asf1–histone H3 interaction is reduced in Asf1-185T and 152T mutants. The amount of soluble histones is invariable between the Asf1 mutant strains used. (A) Western blot analysis of total Asf1 and histone proteins using an H3 C-terminal antibody and an antibody that recognizes H3 acetylated on lysine residues 9 and 14 was performed with soluble protein extracts from yeast strains ACN026 (WT), ROY1172 (no tag), BAT004 (Asf1-185T), and BAT006 (Asf1-152T). The amount of total protein loaded from each sample was 7.5 mg/ml. (B) Co-immunoprecipitation analysis of histones with Asf1. Yeast strains used in A were immunoprecipitated with an anti-myc antibody and Western blot analysis was used to visualize H3 and Rad53. “Crosslinker” indicates the use of a protein–protein crosslinker, DSP. Newly synthesized soluble histones are acetylated on lysine 9 of histone H3; therefore, the anti-H3 acetyl lysine 9/14 antibody was used to detect all soluble histones bound to Asf1, as used previously (Tamburini et al. 2005).