Abstract
Neuronal–glial communication is essential for constructing the orthogonal axon scaffold in the developing Drosophila central nervous system (CNS). Longitudinal glia (LG) guide extending commissural and longitudinal axons while pioneer and commissural neurons maintain glial survival and positioning. However, the transcriptional regulatory mechanisms controlling these processes are not known. Previous studies showed that the midline function of the jing C2H2-type zinc-finger transcription factor was only partially required for axon scaffold formation in the Drosophila CNS. We therefore screened for gain-of-function enhancers of jing gain of function in the eye and identified the Drosophila homolog of the disease gene of human α-thalassemia/mental retardation X-linked (ATR-X) as well as other genes with potential roles in gene expression, translation, synaptic transmission, and cell cycle. jing and DATR-X reporter genes are expressed in both CNS neurons and glia, including the LG. Coexpression of jing and DATR-X in embryonic neurons synergistically affects longitudinal connective formation. During embryogenesis, jing and DATR-X have autonomous and nonautonomous roles in the lateral positioning of LG, neurons, and longitudinal axons as shown by cell-specific knockdown of gene expression. jing and DATR-X are also required autonomously for glial survival. jing and DATR-X mutations show synergistic effects during longitudinal axon formation suggesting that they are functionally related. These observations support a model in which downstream gene expression controlled by a potential DATR-X–Jing complex facilitates cellular positioning and axon guidance, ultimately allowing for proper connectivity in the developing Drosophila CNS.
DURING central nervous system (CNS) development, axons navigate long distances and are faced with both attractive and repulsive guidance cues that must be properly interpreted (Tessier-Lavigne and Goodman 1996). Many interneurons, whose cell bodies lie next to the CNS midline, must project their axons across the midline to form the commissural tracts. The “decision” of an axon to cross the midline of the Drosophila ventral nerve cord (VNC) and the vertebrate spinal cord depends on the differential response of axons to the midline repellent Slit and to the attractant Netrins (Seeger et al. 1993; Tessier-Lavigne 1994; Battye et al. 1999; Kidd et al. 1999; Long et al. 2004; Bhat 2005). After commissural axons cross the midline, they turn to fasciculate with the longitudinal tracts that run parallel to the midline and are repelled from the midline by Slit.
The ligand of Slit is Roundabout (Robo), which is located on the longitudinal glia (LG) and associated pioneer neuron growth cones adjacent to the midline (Kidd et al. 1998a; Kinrade et al. 2001). Signaling and cell–cell contact maintain the ipsilateral positions of both LG and connectives. In fact, Slit–Robo signaling cancels out the attraction of longitudinal axons to the CNS midline by Netrin–Frazzled (Bhat 2005). Commissureless (Comm) is a transmembrane protein that prevents the delivery of Robo to the growth cones, specifically in commissural neurons, allowing their axons to cross the midline (Tear et al. 1996; Keleman et al. 2002; Keleman et al. 2005). A downregulation of Robo by genetic means or by overexpression of comm results in an excess of axons at the CNS midline (Kidd et al. 1998b). Therefore, the differential localization of Comm, Robo, and Slit determines what directions navigating axons of the scaffold will follow. The Slit–Robo system is an important and conserved mechanism to establish cellular positioning and boundaries in the developing vertebrate and invertebrate nervous systems (Kidd et al. 1999; Rajagopalan et al. 2000a,b; Simpson et al. 2000a,b; Rasband et al. 2003; Barresi et al. 2005).
The relationship between neurons and glia and the formation of the Drosophila CNS axon tracts has been extensively studied by genetic and cell ablation methods (Hidalgo and Brand 1997, 2000; Booth et al. 2000). The longitudinal axon tracts are constructed by the extensions of four pioneer neurons (Bate and Grunewald 1981; Jacobs and Goodman 1989; Hidalgo and Brand 1997). To form a longitudinal fascicle, the dMP2 and MP2 pioneer neurons extend their axons posteriorly to contact the anteriorly projecting growth cones of the vMP2 and pCC neurons (Jacobs and Goodman 1989). In each hemisegment, LG act as mobile guideposts for the migrating axons (Hidalgo and Booth 2000). Early ablation of LG affects the joining of the descending and ascending pioneer growth cones and the subsequent fasciculation and defasciculation of pioneer and later follower axons (Booth et al. 2000; Hidalgo and Booth 2000). Despite the important guidance role of the LG, these cells depend on pioneer axons for their survival (Hidalgo et al. 2001; Kinrade et al. 2001). In addition, contralateral neuron cell bodies are needed for axon pathfinding onto the longitudinal connective (Whitington et al. 2004). The proper migration of follower glia in the fly optic lobe requires a preexisting photoreceptor axon scaffold (Hidalgo et al. 2001; Kinrade et al. 2001; Dearborn and Kunes 2004). Therefore, neuronal–glial interactions, in addition to guidance molecules, are instrumental during axon patterning (Oland and Tolbert 2002).
The jing gene was originally identified in two genetic screens for regulators of border cell migration in the ovary and for midline cell development during embryogenesis (Liu and Montell 2001; Sedaghat et al. 2002). During embryogenesis, jing transcripts accumulate in the CNS midline, adjacent neuroectoderm, brain, and trachea (Sedaghat et al. 2002; Sonnenfeld et al. 2004). In the CNS midline and trachea, Jing functions downstream of basic helix-loop-helix and PAS (Per–Arnt–Sim)-containing (bHLH-PAS) transcription factors to control tyrosine kinase signaling through the epidermal growth factor receptor (Egfr) and fibroblast growth factor receptor Breathless (Sedaghat et al. 2002; Sonnenfeld et al. 2004). In the CNS midline, jing is required for commissural and longitudinal axon formation but midline expression of wild-type jing does not completely rescue axon defects in mutants, suggesting that other functions of Jing contribute to axon tract formation (Sonnenfeld et al. 2004).
In a search for additional factors important for jing function, we carried out a genetic screen to identify genes whose function could modify that of jing in a gain-of-function (GOF) assay in the developing eye-imaginal disc. Seven third chromosome enhancer/promoter-tagged (EP) genes were identified whose GOF enhanced that of jing. This group of genes specifically interacts with jing and each other during ommatidial formation. Of these, we identified the Drosophila homolog of the disease gene of human α-thalassemia/mental retardation (MR) X-linked (DATR-X) (Gibbons et al. 1995b). The human ATR-X gene encodes a zinc-finger ATPase that is involved in chromatin remodeling and is the disease gene of several MR syndromes (Gibbons et al. 1995b; Villard et al. 1996a,b; Abidi et al. 1999; Xue et al. 2003; Tang et al., 2004).
To explore the transcriptional mechanisms controlling axon patterning, we investigated the cell-specific roles of jing and DATR-X in regulating axon formation in the embryonic CNS. The roles of DATR-X and jing in the CNS were studied by reducing their expression specifically in neurons or glia using RNA interference (RNAi) and by analyzing the GOF effects of each gene. We show that jing and DATR-X have (1) autonomous roles in CNS glial survival, (2) autonomous and nonautonomous roles in LG and axon positioning, and (3) autonomous and nonautonomous roles in longitudinal axon outgrowth. The phenotypes of jing and DATR-X mutations derive from a perturbation in the extensive neuronal–glial communication mechanisms that govern CNS axon scaffold formation. Early ubiquitous expression of DATR-X mRNA and an enrichment of transcripts and reporter gene expression in embryonic neurons and glia reveal a similarity to the expression pattern of jing (Sedaghat et al. 2002). The combined effects of jing and DATR-X knockdown and GOF are synergistic and the phenotypes resulting from single gene knockdown are similar, providing strong evidence that jing and DATR-X may work together. Therefore, jing and DATR-X function is instrumental in the neuronal–glial communication mechanisms that govern CNS axon scaffold formation.
MATERIALS AND METHODS
Drosophila strains:
All flies were raised on standard Drosophila cornmeal medium at 25° (Ashburner 1989). The collection of third chromosome transgenic EP strains, generated and described by Rørth (1996), was obtained from the Hungarian Szeged stock center. GMR-Gal4 (second chromosome) was obtained from J. Nambu (Hay et al. 1994; Wing et al. 2002) and paired (prd)-Gal4 (third chromosome) were obtained from S. Crews. The driver, ELAV-Gal4 (on X), was used to drive expression in neurons and is a promoter fusion of the embryonic lethal abnormal vision gene (Robinow and White 1988; Yao and White 1994); it was obtained from the Bloomington stock center. GCM-Gal4 drives expression in all CNS glia expressing the glial cells missing (GCM) gene and was obtained from M. Freeman (Freeman et al. 2003; Hosoya et al. 1995; Jones et al. 1995;). btl-Gal4 was used to drive expression in the trachea (Shiga et al. 1996).
UAS-jingE, jing EMS alleles, and the jing deficiency [Df (2R)ST1] were previously described (Sedaghat et al. 2002; Sonnenfeld et al. 2004). UAS-jingU is a second chromosome insertion that expresses jing as determined by in situ hybridization.
Genetic crosses:
Balancers were detected using Cyo wingless-lacZ or TM3 ubx-lacZ. DATR-X and jing were overexpressed in CNS neurons by crossing homozygous ELAV-Gal4 with homozygous UAS-jingE, EP(3)0635, or UAS-DATR-X. For coexpression experiments, flies carrying ELAV-Gal4 and UAS-jingE were crossed with flies homozygous for EP(3)0635. Controls included ELAV-Gal4/+, UAS-jingE/+, and EP(3)0635/+. For double RNAi flies carrying ELAV-Gal4 and UAS-jing RNAi were crossed to flies carrying UAS-DATR-XRNAi.
EP screen:
Males from each EP line were crossed to virgin females carrying GMR-Gal4. The eye morphologies of F1 male progeny from this cross were analyzed under a dissecting microscope and male flies with straight wings were then crossed to virgin females carrying UAS-jingE. One copy of UAS-jingE is not associated with a rough eye phenotype and therefore any rough eye phenotype in combination with a particular EP line and GMR-Gal4 represented an interaction. The number of flies with rough eyes was determined under a dissecting microscope where at least 600 progeny were analyzed in each of three trials at 25°. Controls included in each trial included GMR-Gal4/UAS-jingE and GMR-Gal4/+ and did not show a rough eye at 25°. As an additional control, eyes were inspected from flies heterozygous for UAS-jingE and the enhancing EP line. Enhancing lines were subjected to three more genetic trials with GMR-Gal4 and UAS-jingE at 25°. The penetrance was determined as the number of rough eyes divided by the 25% that carried GMR-Gal4, UAS-jingE, and the particular EP strain. A total of 591 individual third chromosome EP lines were screened. Enhancer EP lines were tested for interactions inter se and with additional EP lines expressing DNA regulatory genes and random EP lines by the same method as described above.
Microscopy:
For scanning electron microscopy (SEM), 1- to 3-day-old adult heads were dissected, fixed, and dehydrated by 15-min incubations in a graded ethanol series. Dehydrated heads were sent to the Mount Sinai Bioimaging Center (Toronto) for sputter coating with gold-palladium and SEM examination. Three eyes from each sample were examined and processed using Adobe Photoshop software. For light microscopy, 1- to 3-day-old adult heads were dissected, analyzed on a Zeiss Axioskop, and images were captured on a Nikon DXM1200 digital camera and processed using Adobe Photoshop software. Confocal microscopy was carried out on a BioRad1024 microscope using ×40 water immersion [1.15 numerical aperture (NA)] or ×20 (1.1 NA) objectives.
Antibodies and immunostaining of Drosophila embryos:
The following antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA): BP102 (1:10), anti-Repo (8D12, 1:10) (Campbell et al. 1994), 1D4 (Fasciclin II, 1:10)(Van Vactor et al. 1993), and anti-Robo (13C9, 1:10) (Kidd et al. 1998a). A rabbit antibody against β-galactosidase (Promega) was used at 1:100 dilution. HRP-conjugated goat anti-mouse IgG was used at 1:100. Texas red and FITC-conjugated secondary antibodies were used for confocal microscopy. Monoclonal antibody 2A12 was used to visualize tracheal tubules (1:3).
Embryos were fixed in PBS containing 4% formaldehyde and embryos were incubated overnight with primary antibodies in PTN (1× PBS, 0.1% Triton X-100, 5% normal goat serum). Horseradish peroxidase-conjugated secondary antibodies were used with H2O2/diaminobenzedine histochemistry. Stained embryos were dehydrated through an ethanol series, mounted in methyl salicylate, and examined by Nomarski optics on a Zeiss Axioskop microscope. Images were captured on a Nikon DXM1200 digital camera and processed using Adobe Photoshop software.
Molecular genetic analysis of DATR-X and generation of UAS lines:
Genomic and cDNA sequences encoding the Drosophila DATR-X homolog were identified by searching the Berkeley Drosophila Genome Project (BDGP) database for the EP(3)0635 associated gene (Adams et al. 2000; Rubin et al. 2000). The location of the EP element in EP(3)0635 was confirmed by PCR using inverted repeat primers and flanking genomic DATR-X sequence (CGACGGGACCACCTTATGTTATTTCATCATG) followed by DNA sequence analysis. DATR-X cDNAs [expressed sequence tags (ESTs) SD07188 and LD28477] were obtained from Open Biosystems and jing cDNA was previously obtained from Research Genetics (Sedaghat et al. 2002). DATR-X EST SD07188 contains 2382 bp of DATR-X coding sequence beginning at base pair 1968 with respect to full-length LD28477 cDNA sequence. DATR-X clones SD07188 and LD28477 were subjected to DNA sequence analysis and compared to that in the Gadfly database (Celera genomics) (Adams et al. 2000). Homology searches were done using BLAST (http://www.ncbi.nlm.nih.gov). Full-length DATR-X sequence (accession AAL13821; FlyBase) was aligned with that of Caenorhabditis elegans (AAD55361), mouse (AAC08741), and human (AAB49970) using Clustal X (1.83). Protein sequences were compared using BL2SEQ (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) (Tatusova and Madden 1999). Protein domains were analyzed using SMART and PROSITE (Hulo et al. 2004; Letunic et al. 2004).
Full length DATR-X cDNA (LD28477) was subcloned into the pUAST vector (Brand and Perrimon 1993) as an EcoRI–KpnI fragment and used to generate germline transformant flies by standard procedures (Genetic Services, Cambridge, MA) (Rubin and Spradling 1983). Expression of DATR-X was confirmed in multiple lines by in situ hybridization on embryos expressing different DATR-X upstream activating sequence transgenes under control of the prd gene transactivator (prd-GAL4).
In situ hybridization and reporter gene construction:
Whole-mount in situ hybridization was carried out using digoxigenin-labeled RNA probes according to Janody et al. (2000). The DATR-X riboprobe was generated by linearizing the SD07188 EST with HaeII. Sense probes were synthesized using the T7 promoter and antisense using Sp6 according to manufacturers' specifications (Roche). Probe specificity was tested in GOF DATR-X embryos carrying the prd-Gal4 driver and EP(3)0635 or UAS-DATR-X transgenes. Third instar larval nerve cords were dissected in PBS and fixed in 4% formaldehyde for 20 min. Fixed nerve cords were rinsed three times in fresh PBS and washed for 1 hr. Nerve cords were then subjected to in situ hybridization as described above. Digoxigenin-stained nerve cords and embryos were mounted in 80% glycerol and examined on a Zeiss Axioskop 2.
A total of 1553 bp from the 5′ region of jing were obtained by PCR using Drosophila w1118 genomic DNA as template, sequenced, and cloned into the KpnI and NotI sites of the pCaSper vector for in vivo expression. A total of 593 bp were obtained by PCR from the 5′ DATR-X region surrounding the EP element insertion site. This fragment was cloned into the EcoRI site in pCaSper. To generate transgenic strains, the pCaSper plasmids were co-injected together with a plasmid encoding Δ2-3 transposase into w1118 embryos by P-element-mediated transformation (Genetic Services) (Rubin and Spradling 1983). Multiple strains were analyzed for lacZ expression.
RNA interference:
For DATR-X RNAi, a 697-bp sequence was generated by PCR from base pair positions 3181–3837 using LD28477 cDNA as a template. For RNAi of jing, a 597-bp sequence was generated by PCR from base pair position 2289 from the ATG on the LD36562 cDNA. The DNA fragments were individually subcloned sequentially, in opposite directions, into the AvrII and NheI sites of the pWIZ plasmid (a gift of R. Carthew) except that the inverted sequence was directionally cloned using XbaI and NheI restriction enzymes (Lee and Carthew 2003). The final inverted repeat containing vectors UAS-DATR-X RNAi and UAS-jing RNAi was confirmed by sequence analysis. To generate transgenic strains, the UAS-DATR-X RNAi or UAS-jing RNAi plasmids, were co-injected together with a plasmid encoding Δ2-3 transposase into w1118 embryos by P-element-mediated transformation (Genetic Services) (Rubin and Spradling 1983).
Several independent UAS-DATR-X RNAi and UAS-jing RNAi transformant lines were bred to homozygosity, mapped, and tested for double-stranded RNAi activity by crossing to CNS- (ELAV-Gal4) and tracheal-specific Gal4 (btl-Gal4) drivers and immunostaining with monoclonal antibodies 1D4 and 2A12, respectively. To confirm downregulation of mRNA expression, embryos carrying armadillo-Gal4 (Ahmad and Henikoff 2001) and UAS-DATR-X RNAi or UAS-jing RNAi were subjected to in situ hybridization using DATR-X or jing riboprobes, respectively. The number of stage 13 embryos with reduced expression in transgene- and Gal4 driver-carrying embryos was compared with that in control embryos carrying either the driver or the transgene alone.
Quantitative analysis of longitudinal axon and glial cell defects:
The number of crossovers per segment was counted in stage 16/17 control embryos and those expressing multiple jing or DATR-X RNAi transgenes were driven either by ELAV-Gal4 or by GCM-Gal4 and stained with 1D4 and anti-Robo. Four independent transgenic lines and controls were analyzed. Neuronal expression of pWiz-DATR-X022, pWiz-DATR-X28-MO1, pWiz-DATR-X26-MO2, and pWiz-DATR-X28-MO2 resulted in 20.9% (n = 200), 22% (n = 178), 27% (n = 187), and 30% (n = 145) of embryos with axon defects, respectively. Neuronal expression of pWiz-jingNC1A, pWiz-jingORO3, pWiz-jingNA2, pWiz-jing006 resulted in 25% (n = 180), 29% (n = 210), 28% (n = 168), and 31% (n = 201) of embryos with axon defects, respectively.
Quantitative analysis of glia was carried out by light microscopy on anti-Repo stained whole-mount embryos from stage 11 to 16. Glia were counted in each nerve cord segment of embryos expressing four independent jing or DATR-X RNAi transgenes as driven either by ELAV-Gal4 or by GCM-Gal4. Controls included embryos heterozygous for each RNAi transgene, the Gal4 driver as well as w1118. Standard deviations in glial numbers in each sample were determined using Microsoft Excel and sample sizes exceeded 250 nerve cord segments. Differences in the number of glia in each sample relative to controls were evaluated for significance using Student's paired t-test. Any space in the longitudinal connectives was considered a longitudinal break.
RESULTS
Identification of the Drosophila homolog of human ATR-X and additional genes involved in jing function:
Our approach to identify genes interacting with jing was to use the EP collection of strains including genes tagged by insertion of a transposable element containing UAS sites (Szeged stock center, Szeged, Hungary) (Brand and Perrimon 1993; Rørth 1996). We reasoned that jing GOF may show dosage effects in the larval eye that could be easily scored in the adult since this neural tissue is sensitive to studies of transcriptional regulatory mechanisms (Rubin 1988; Zipursky and Rubin 1994) and jing has a neuronal function (Sedaghat et al. 2002).
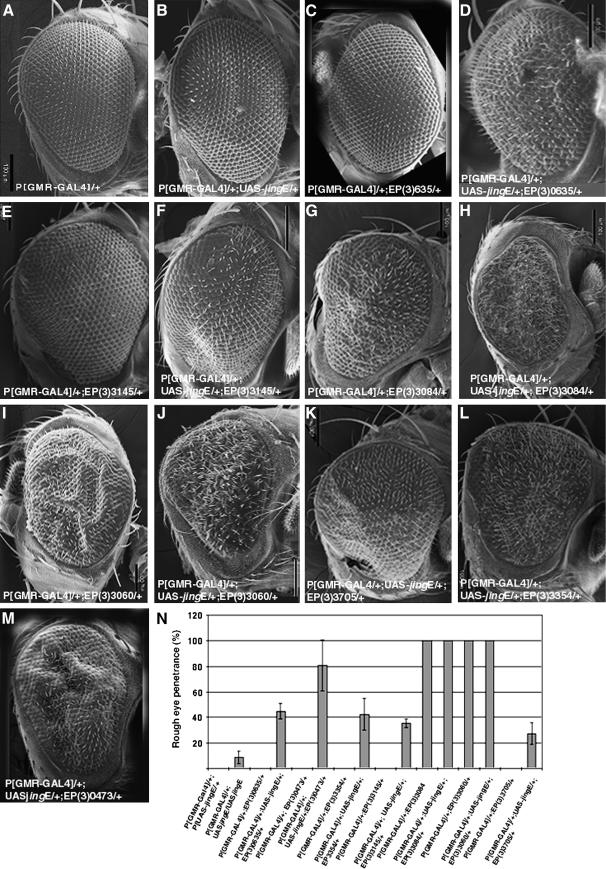
An enhancer screen was carried out using the third chromosome collection of EP lines (see materials and methods) (Rørth 1996). Of 591 third-chromosome EP lines screened, 7 were found to repeatedly interact genetically when coexpressed with jing in the eye under control of GMR-Gal4 (Table 1; Figure 1). Eye-specific expression of one copy of a UAS-jing transgene has no visible effects on ommatidial formation (Figure 1B) as compared with eyes heterozygous for GMR-Gal4 (Figure 1A). However, coexpression of jing with EP(3)0635, which controls expression of the Drosophila homolog of human ATR-X (DATR-X), disrupted ommatidial formation (Figure 1D). Eye-specific expression of EP(3)0635 alone had no effect (Figure 1C). jing also interacted with 4 other EP lines, including EP(3)3145, EP(3)3705, EP(3)3354, and EP(3)0473, resulting in rougher eyes (Figure 1, F, K–M). A loss in pigmentation occurred in the case of EP(3)3354. These lines had no effect when expressed alone in the eye (Figure 1N; Table 2).
TABLE 1.
EP lines that enhanced jing gain of function
| EP line | Gene | Predicted function |
|---|---|---|
| EP(3)0635 | ATR-X/XNP | Chromatin remodeling |
| EP(3)0473 | D1 | Chromatin remodeling |
| EP(3)3145 | Dataxin-2 | Translational regulation |
| EP(3)3060 | lap | Receptor-mediated endocytosis |
| EP(3)3705 | CG32137 | Microtubule binding (cell cycle) |
| EP(3)3354 | CG17383 (JIGR-1) | Transcriptional regulation |
| EP(3)3084 | CG15507 | Unknown |
Rough eyed flies were scored in a population carrying GMR-Gal4, UAS-jing, and each EP line under a dissecting microscope. Crosses were repeated at least three times at 25° totaling a sample size of >1200 flies.
Figure 1.—
Identification of enhancer/promoter (EP) lines that enhance jing gain –of function in the eye. (A–M) Scanning electron micrographs of adult eyes of the following genotypes are shown: (A) GMR-GAL4/+; (B) GMR-GAL4/+; UAS-jingE/+; (C) GMR-GAL4 /+; EP(3)0635/+; (D) GMR-GAL4/+; UAS-jingE /+; EP(3)0635/+; (E) GMR-GAL4/+; EP(3)3145/+; (F) GMR-GAL4/+; UAS-jingE /+; EP(3)3145/+; (G) GMR-GAL4/+; EP(3)3084/+; (H) GMR-GAL4/+; UAS-jingE /+; EP(3)3084/+; (I) GMR-GAL4/+; EP(3)3060/+; (J) GMR-GAL4/+; UAS-jingE /+; EP(3)3060/+; (K) GMR-GAL4/+; EP(3)3705 /+; UAS-jingE/+; (L) GMR-GAL4/+; UAS-jingE /+; EP(3)3354/+; (M) GMR-GAL4/+; UAS-jingE /+; EP(3)0473/+. (N) Penetrance values of enhancing phenotypes. Bars, 100 μm.
TABLE 2.
Random screening of jing interacting EP lines
| Eye phenotype | Sample size | Eye phenotype | Sample size | ||
|---|---|---|---|---|---|
| P[GMR-Gal4]- | |||||
| P[GMR-Gal4] × | EP(3)0473 × | ||||
| EP(3)3339 | Wild type | 250 | EP(3)3339 | Rough | 200 (60) |
| EP(3)3303 | Wild type | 178 | EP(3)3303 | Rough | 250 (40) |
| EP(3)3258 | Wild type | 189 | EP(3)3258 | Rough | 214 (37) |
| EP(3)3916 | Wild type | 201 | EP(3)3916 | Rough | 210 (23) |
| EP(3)893 | Wild type | 200 | EP(3)893 | Rough | 276 (16) |
| EP(3)3377 | Wild type | 215 | EP(3)3377 | Rough | 234 (42) |
| P[GMR-Gal4]- | P[GMR-Gal4]- | ||||
| EP(3)3084 × | Glossy, ↓pigment | 400 | EP(3)3060 × | Rough | 450 |
| EP(3)3339 | No change | 260 | EP(3)3339 | Enhanced | 76 (20) |
| EP(3)3303 | No change | 201 | EP(3)3303 | Enhanced | 60 (53.3) |
| EP(3)3258 | No change | 192 | EP(3)3258 | Enhanced | 60 (6.7) |
| EP(3)3916 | No change | 189 | EP(3)3916 | No change | 150 |
| EP(3)893 | No change | 234 | EP(3)893 | No change | 200 |
| EP(3)3377 | No change | 250 | EP(3)3377 | Enhanced | 160 (75) |
| P[GMR-Gal4]; | P[GMR-Gal4]; | ||||
| EP(3)0635 × | Wild type | 650 | EP(3)3145 × | Wild type | 630 |
| EP(3)3339 | Wild type | 251 | EP(3)3339 | Wild type | 151 |
| EP(3)3303 | Wild type | 234 | EP(3)3303 | Wild type | 145 |
| EP(3)3258 | Wild type | 243 | EP(3)3258 | Wild type | 130 |
| EP(3)3916 | Wild type | 267 | EP(3)3916 | Wild type | 125 |
| EP(3)893 | Wild type | 154 | EP(3)893 | Mild rough | 218 (6.4) |
| EP(3)3377 | Wild type | 134 | EP(3)3377 | Wild type | 245 |
| P[GMR-Gal4]; | P[GMR-Gal4]; | ||||
| EP(3)3354 × | Wild type | 550 | EP(3)3705 × | Wild type | 601 |
| EP(3)3339 | Wild type | 200 | EP(3)3339 | Wild type | 160 |
| EP(3)3303 | Wild type | 250 | EP(3)3303 | Wild type | 134 |
| EP(3)3258 | Wild type | 214 | EP(3)3258 | Wild type | 191 |
| EP(3)3916 | Wild type | 210 | EP(3)3916 | Wild type | 230 |
| EP(3)893 | Wild type | 276 | EP(3)893 | Wild type | 212 |
| EP(3)3377 | Wild type | 234 | EP(3)3377 | Wild type | 213 |
Sample sizes include the total number of progeny. Sample sizes for EP lines with a phenotype, such as EP(3)3060, EP(3)3084, and EP(3)0473, include the number of eyes with a phenotype. Percentages in parentheses indicate the penetrance as the number of enhanced eyes over the total number of eyes with a phenotype.
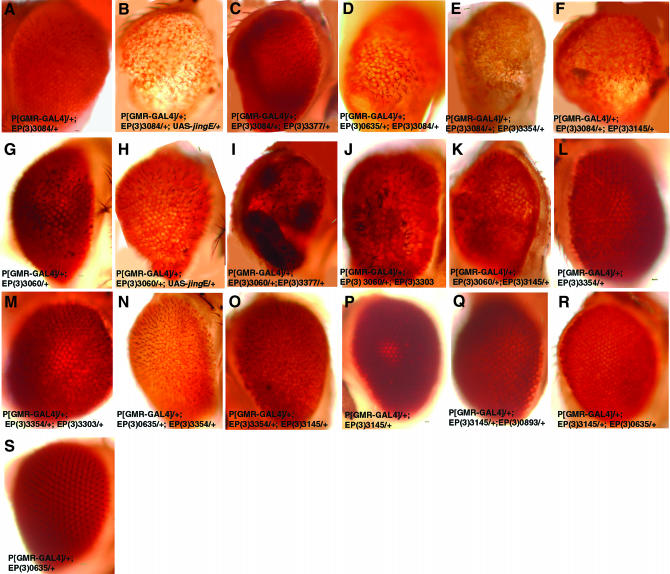
Two additional EP lines that interacted with jing caused a rough eye phenotype when expressed alone in the eye, including EP(3)3084 (Figure 1G) and EP(3)3060 (Figure 1I). GMR-Gal4-driven expression of EP(3)3084 was associated with morphological defects, mild reductions in pigmentation, and glossy eyes in 100% of flies (Figure 1, G and N; Figure 2A). However, coexpression of EP(3)3084 with jing resulted in an enhancement of this phenotype in which eye size was reduced, eyes were rougher, and pigmentation was completely lost (Figure 1H; Figure 2B). A total of 35% of eyes with a phenotype were enhanced (n = 345). Eye-specific expression of EP(3)3060 caused a mild rough eye (Figure 1I) with no loss in pigmentation (Figure 2G). Enhancement of this phenotype was observed after EP(3)3060 and jing coexpression, which included rougher eyes (Figure 1J) and loss in pigmentation (Figure 2H). A total of 38% of eyes with a phenotype were enhanced (n = 301).
Figure 2.—
Genetic interactions of jing enhancers. Light level images of adult eyes of the following genotypes are shown: (A) GMR-GAL4 /+; EP(3)3084/+; (B) GMR-GAL4/+; EP(3)3084/+; UAS-jingE/+; (C) GMR-GAL4/+; EP(3)3084/+; (D) EP(3)3377/+; GMR-GAL4/+; EP(3)0635/+; EP(3)3084/+; (E) GMR-GAL4/+; EP(3)3084/+; EP(3)3354/+; (F) GMR-GAL4/+; EP(3)3084/+; EP(3)3145/+; (G) GMR-GAL4/+; EP(3)3060/+; (H) GMR-GAL4/+; EP(3)3060/+; UAS-jingE/+; (I) GMR-GAL4/+; EP(3)3060/+; EP(3)3377/+; (J) GMR-GAL4/+; EP(3)3060/+; EP(3)3303/+; (K) GMR-GAL4/+; EP(3)3060/+; EP(3)3145/+; (L) GMR-GAL4/+; EP(3)3354/+; (M) GMR-GAL4/+; EP(3)3354/+; EP(3)3303/+; (N) GMR-GAL4/+; EP(3)0635/+; EP(3)3354/+; (O) GMR-GAL4/+; EP(3)3354/+; EP(3)3145/+; (P) GMR-GAL4/+; EP(3)3145/+; (Q) GMR-GAL4/+; EP(3)3145/+; EP(3)0893/+; (R) GMR-GAL4/+; EP(3)3145/+; EP(3)0635/+; (S) GMR-GAL4/+; EP(3)0635/+. Bars, 50 μm.
Specificity of EP interactions:
The genetic synergy between jing and the enhancers suggests that these genes may function in similar processes in neural cells. However, the group of jing-interacting genes are involved in a wide range of functions, including chromatin remodeling (DATR-X and D1) (Rodriguez-Alfageme et al. 1980; Ashley et al. 1989; Gibbons et al. 1995b; Picketts et al. 1996), transcriptional regulation [EP(3) 3354] (Ewel et al. 1990; England et al. 1992; Cutler et al. 1998; Brody et al. 2002; Bhaskar and Courey 2002), translational regulation [EP(3)3145, Datx2] (Satterfield et al. 2002; Ciosk et al. 2004), synaptic vesicle transport [EP(3)3060] (Pena-Rangel et al. 2002), and cell cycle control [EP(3)3705] (BDGP). To test the specificity of the interactions with jing, the enhancers were crossed with six random EP lines, including EP(3)3339, EP(3)3303, EP(3)3258, EP(3)3916, EP(3)893, and EP(3)3377, and were driven in the eye with GMR-Gal4. Of the enhancers, only EP(3)3060 and EP(3)0473 interacted with multiple EP lines (Table 2; Figure 2, I and J). In addition, EP(3)3145 interacted with EP(3)0893, resulting in mildly rough eyes (Table 2).
Next, we addressed the specificity of D1 and DATR-X enhancement by testing for interactions with other regulatory genes in the EP collection. DATR-X [EP(3)635] did not interact with other EP elements upstream from genes encoding DNA-binding proteins, including EP(3)1005 (n = 411) and EP(3)1096 (n = 387). The P element in line EP(3)1005 lies upstream from CG15514, which encodes a protein containing a DNA-binding BED finger found in chromatin-boundary-element-binding proteins and transposases (FlyBase) (Aravind 2000). The P element in line EP(3)1096 lies upstream of a gene encoding a CXXC zinc finger (FlyBase, CG11033). EP(3)0635 did not interact with EP(3)3058 (n = 200), which lies upstream from the poly U binding factor 68kD (pUf68) gene (Page-McCaw et al. 1999; Lasko 2000). However, EP(3)0635 did interact with EP(3)3205 (35% penetrance, n = 301), which lies upstream of CG7552 encoding a protein with a WW domain and interacts genetically with cyclin E (Tseng and Hariharan 2002). In contrast, EP(3)0473 interacted with EP(3)1005 (48%, n = 205), EP(3)3205 (12%, n = 150), and EP(3)3058 (83%, n = 256), indicating that it may be a more general transcriptional regulator.
Interenhancer interactions:
Last, we determined if the jing enhancers interacted with each other in the eye. Eye-specific coexpression of EP(3)3084 with EP(3)0635, EP(3)3354, and EP(3)3145 resulted in more severe rough eye phenotypes including losses in pigmentation (Figure 2, D–F; Table 3). Eye-specific expression of EP(3)3060 resulted in rougher eyes when coexpressed with EP(3)3145, EP(3)3354, EP(3)0635, EP(3)0473, and EP(3)3705 (Figure 2K; Table 3). However, the widespread interactions of EP(3)3060 suggest that these results may be considered nonspecific (Table 2; Figure 2, I and J).
TABLE 3.
Screening of jing interacting EP lines
| P[GMR-Gal4]; EP(3)3145 | Eye phenotype | % penetrance | N | P[GMR-Gal4]; EP(3)0635 | Eye phenotype | % penetrance | N |
|---|---|---|---|---|---|---|---|
| EP(3)0635 | Glossy, ↓↓ pigment | 17.9 | 217 | EP(3)3084 | ↓↓↓ pigment | 46.1a | 256 |
| EP(3)3060 | Bumpy, ↓ pigment | 49.0a | 300 | EP(3)3354 | Glossy, ↓↓↓ pigment | 17.2 | 93 |
| EP(3)3354 | Glossy, ↓↓ pigment | 14.1 | 85 | EP(3)3060 | Glossy, ↓↓ pigment | 88.9a | 90 |
| EP(3)3084 | Rougher, ↓↓↓ pigment | 30.8a | 65 | EP(3)3705 | Rough | 20 | 200 |
| EP(3)3705 | Rough | 8.5 | 94 | EP(3)0473 | Rough | 18.8 | 223 |
| EP(3)0473 | Rough | 15.0 | 167 | ||||
| P[GMR-Gal4]; EP(3)3084 | Eye phenotype | % penetrance | N | P[GMR-Gal4]; EP(3)3060 | Eye phenotype | % penetrance | N |
| EP(3)3060 | No change | 0 | 250 | EP(3)3354 | Smaller, rougher | 40a | 70 |
| EP(3)3354 | Glossy, ↓↓↓ pigment | 21.4a | 234 | EP(3)473 | Rougher | 7.5a | 90 |
| EP(3)0473 | Rougher | 24.9a | 201 | EP(3)3705 | Smaller, rougher | 18.7a | 75 |
| EP(3)3705 | Rougher | 20.8a | 120 | ||||
| P[GMR-Gal4]; EP(3)3354 | Eye phenotype | % penetrance | N | ||||
| EP(3)3705 | Small | 18.4 | 250 | ||||
| EP(3)0473 | Rough | 15.6 | 225 |
Penetrance is indicated by percentage of the eyes with a phenotype out of the progeny carrying GMR-Gal4 and the EP elements. N, sample sizes.
Penetrance for EP lines with a phenotype such as EP(3)3060, EP(3)3084, and EP(3)0473 is the percentage of the enhanced eyes out of the total eyes with a phenotype.
Eye-specific coexpression of EP(3)3354 with EP(3)3145 and EP(3)0635 resulted in glossy eyes with severely reduced pigmentation representing synergistic interactions (Table 3; Figure 2, N and O). We have designated EP(3)3354 as jing interacting gene regulatory 1 (JIGR1), given its potential role in regulating gene expression (Brody et al. 2002). The EP element in JIGR1 lies upstream of the transcript CG17383, which was identified in a differential head cDNA screen (Brody et al. 2002). JIGR1 contains an MADF domain shown in the Adf-1 transcription activator to bind DNA specifically to several developmentally regulated Drosophila gene promoters (Ewel et al. 1990; England et al. 1992; Cutler et al. 1998; Bhaskar and Courey 2002). JIGR1 also interacted with EP(3)3705 resulting in a reduced eye size (Table 3).
EP(3)3145 lies upstream of Drosophila ataxin2 (Datx2) and interacted with other jing enhancers from the screen but not with most randomly chosen EP lines. We observed glossy eyes and reduced pigmentation after coexpression of EP(3)3145 with EP(3)635 and EP(3)3354 (Figure 2, R and O). Collectively, these results identify a group of genes that have related functions pertaining specifically to jing and each other during eye development.
Part of ATR-X is highly conserved:
Previous studies showed that ATR-X is involved in gene regulation by chromatin remodeling functions (Cardoso et al. 1998; Xue et al. 2003; Tang et al. 2004). Given the GOF interactions between DATR-X and jing in the eye, we proposed that there may be a regulatory relationship between DATR-X and Jing in the embryonic CNS and initiated further studies of DATR-X. Database searches with genomic sequence flanking the EP(3)0635 P element confirmed that the transposon in the strain we had obtained was inserted in the 5′-untranslated region of the predicted gene CG4548 encoding the Drosophila homolog of human XNP/DATR-X (GadFly).
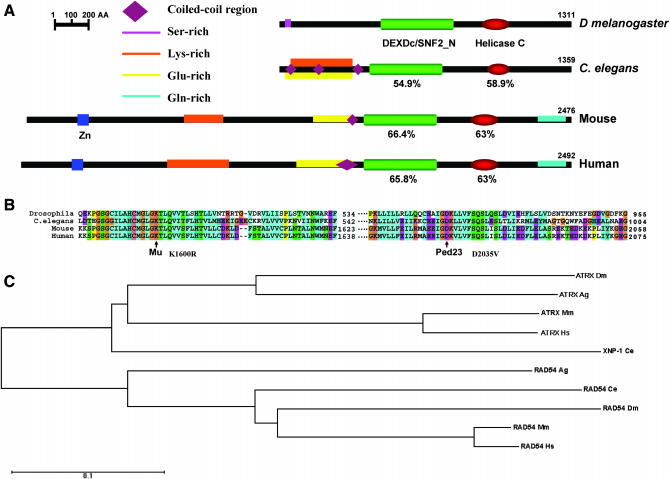
DATR-X is located at cytological interval 96E1 on the third chromosome (BDGP) and encodes a predicted polypeptide of 1311 amino acids with 49.4% overall similarity to that of human ATR-X by alignment using the Clustal X algorithm (Figure 3A). ATR-X homologs are found in human, mouse, C. elegans, planarians, and Drosophila (Figure 3, A and C) (Villard et al. 1999). Most of the sequence conservation between human and DATR-X is confined to the helicase and SNF2 domains. The SNF2 domains of C. elegans, mouse, and human ATR-X show 54.9, 66.4, and 65.8% amino acid identity with that of DATR-X, respectively (Figure 3A). The ATPase domains of C. elegans, mouse, and human ATR-X show 58.9, 63, and 63% amino acid identity with that of DATR-X, respectively (Figure 3A). ATR-X has nucleosome-stimulated ATPase activity that is used to modify chromatin structure and that is a common site of patient mutations (Gibbons et al. 1995b; Xue et al. 2003; Tang et al. 2004). Mutations associated with MR are in invariant residues and the amino acids at the sites of two human mutations, designated Mu K1600R and Ped23 D2035V, are conserved in DATR-X (Figure 3B).
Figure 3.—
ATR-X homologs. (A) Comparison of DATR-X protein domain architecture as predicted using SMART and PROSITE. Invertebrate ATR-X is truncated in comparison with mouse and human but shares high identities in the C-terminal amino acids with the latter proteins. Percentage of identity between the SNF2 and helicase C domains of C. elegans, mouse, and human are in comparison to those of D. melanogaster. (B) Comparison of amino acid identity over the regions containing mutations found in the ATPase domain of ATR-X from human patients with ATR-X syndrome. The mutations include both Mu K1600R and Ped23 D2035V in highly conserved regions. (C) Phylogenetic analysis of ATR-X orthologs. The distance between any two sequences is the sum of horizontal branch length separating them. Sequences were aligned using CLUSTALW (Tree View). The sequence residues in each column are colored on the basis of an alignment consensus, which is calculated automatically (for detail color information, refer to CLUSTAL W online help). Species designation follows each protein acronym (Dm, Drosophila melanogaster; Ag, Anopheles gambiae; Mm, murine; Hs, human; Ce, Caenorhabditis elegans). Bar indicates the number of substitutions.
There are some differences in the amino acid sequences of vertebrate and invertebrate ATR-X. Human and mouse ATR-X have a zinc-finger DNA-binding domain consisting of three multicysteine zinc-finger motifs (C2-C2) that is not present in invertebrate ATR-X proteins (Figure 3A) (Cardoso et al. 2000). These zinc fingers are required for nuclear localization and DNA binding by ATR-X (Cardoso et al. 2000). Therefore, it is possible that invertebrate ATR-X proteins require site-specific DNA targeting to carry out their ATPase activities. The C terminal of ATR-X has a region rich in poly glutamine repeats (Figure 3A) proposed to be involved in protein–protein interactions that also is not present in either D. melanogaster or C. elegans ATR-X (Picketts et al. 1996; data not shown). Sequence distances between ATR-X orthologs in different species are schematized in Figure 3C.
DATR-X and jing-lacZ are expressed in CNS glia and neurons:
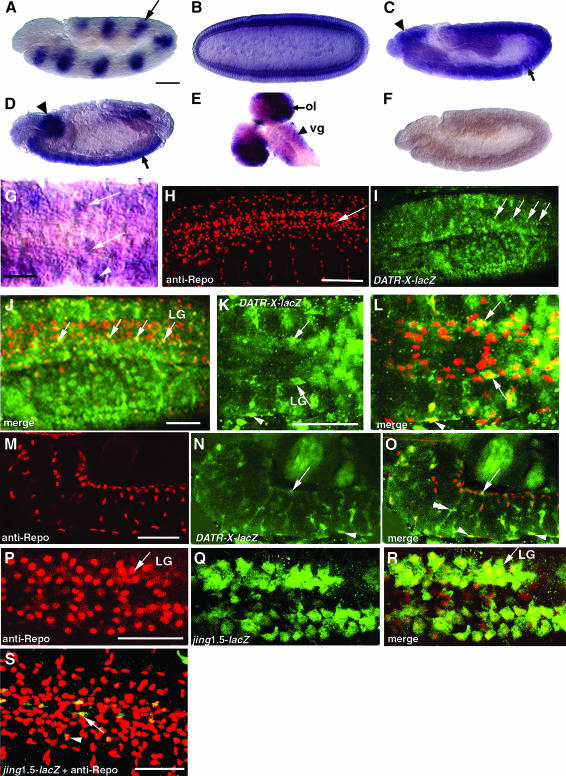
To determine the expression pattern of DATR-X during development, we hybridized whole-mount control embryos with digoxigenin-labeled antisense and sense DATR-X riboprobes. Ectopic activation of EP(3)0635 was detected in prd-expressing stripes with our antisense DATR-X probe confirming the specificity of the probe and EP line (Figure 4A). DATR-X transcripts are present at a low level throughout cellular blastoderm embryos (Figure 4B) and during gastrulation (Figure 4C). In stage 15 embryos, DATR-X transcripts become enriched in the neuroectoderm and supraesophageal ganglion (brain) (Figure 4, D and G). In the mature ventral nerve cord, DATR-X mRNA is expressed in cells along the position of the longitudinal axon tracts (Figure 4G, arrows) and in more laterally located glia (Figure 4G, arrowhead). Similar CNS expression patterns were not observed in embryos hybridized with sense DATR-X riboprobes (Figure 4F). DATR-X transcripts are also present throughout the brains of third instar wild-type larvae but are predominant in the optic lobe region (Figure 4E).
Figure 4.—
Drosophila ATR-X and jing are expressed in CNS glia and neurons. In situ hybridization using a DATR-X digoxigenin-labeled riboprobe and showing wild-type whole-mount embryos with anterior to the left (A–G). Confocal micrographs of stage 15 whole-mount embryos (H–S). Embryos are stained with polyclonal rabbit anti-β-galactosidase (FITC-conjugated secondary antibody, green) and anti-Repo (Texas Red-conjugated secondary antibody, red). (A) To validate the probe specificity, an embryo ectopically expressing DATR-X in ectodermal stripes (arrow) of the paired gene was hybridized with a DATR-X riboprobe. The embryo carries paired-GAL4 and EP(3)0635. (B) DATR-X transcripts are present in cellular blastoderm embryos. (C) A 5.5- to 6-hr-old embryo showing DATR-X transcripts in the supraesophageal ganglion (arrowhead) and neuroectoderm (arrow) in sagittal view. (D) A sagittal stage 15 embryo showing elevated DATR-X transcripts in the supraesophageal ganglion (arrowhead) and ventral nerve cord (arrow). (E) Third instar larval CNS expressing DATR-X in the brain hemispheres (arrow) and ventral ganglion (arrowhead). DATR-X is expressed most strongly in the ventral region of the ganglion (vg, arrowhead) and optic lobe (ol, arrow). (F) In situ hybridization using a DATR-X negative control sense probe. (G) DATR-X expression in a stage 15 ventral nerve cord (ventral view). Strong expression is observed in cells lining the longitudinal connectives (arrows) and in lateral cells (arrowhead). (H–R) Focal planes of DATR-X and jing-lacZ expression in the longitudinal glia (LG) (arrows). (N and O) Sagittal views to show that DATR-X-lacZ is expressed in ventral glia (arrowhead) and the dorsal longitudinal glia (arrow). DATR-X-lacZ is also expressed in neurons (double arrowhead). (P–R) jing-lacZ is strongly expressed in the two rows of longitudinal glial cells. (S) jing-lacZ is also expressed in a bilateral glial lineage (arrow) and in a laterally located lineage (arrowhead) in the ventral focal plane. Bars: (A–F) 200 μm; (G) 30 μm; (H–S) 50 μm.
To better understand the pattern of expression, we costained embryos carrying jing- and DATR-X-lacZ reporter genes with anti-β-galactosidase and the glial-specific anti-Repo antibody. Analysis by confocal microscopy revealed that jing-lacZ and DATR-X-lacZ are expressed in both glial and neuronal lineages. Many of the DATR-X-lacZ- and jing-lacZ-expressing glia are present in positions characteristic of the LG (Figure 4, I–R). Other DATR-X-lacZ- and jing-lacZ-expressing glia occupy more lateral (Figure 4, K and L, arrowheads) or ventral regions (Figure 4, N, O, S).
Pan-neural coexpression of DATR-X and jing synergistically disrupts longitudinal axon formation:
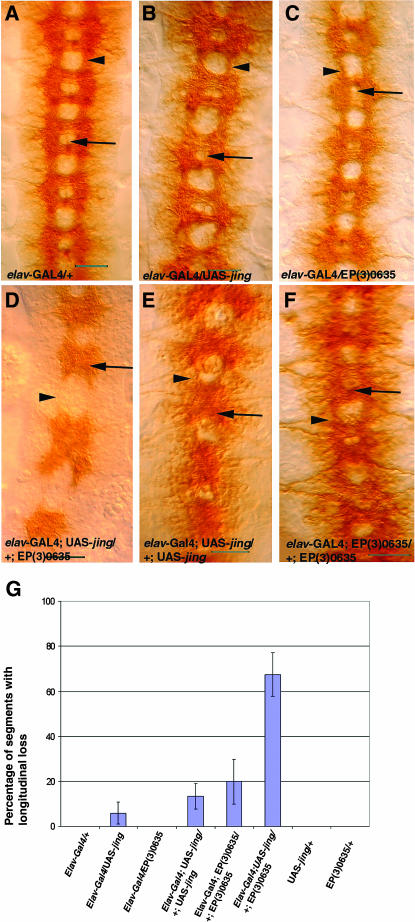
Since coexpression of jing and DATR-X strongly affected adult neuronal development we wanted to determine if their coexpression had similar affects in embryonic neurons. jing and DATR-X were expressed in all postmitotic neurons with ELAV-GAL4. Expression of either jing or DATR-X alone resulted in subtle defects in the CNS axon scaffold as observed with BP102 staining. These defects included thinner longitudinal connectives (Figure 5, B and C, arrowheads) and reduced spacing between the anterior and posterior commissures (Figure 5, B and C, arrows). Additive effects were observed after expression of two copies of either jing (Figure 5E) or DATR-X (Figure 5F) where commissural axons were not properly separated (Figure 5, E and F, arrows).
Figure 5.—
Pan-neural expression of DATR-X and jing in embryonic CNS neurons synergistically affects axon patterning. Ventral views of the ventral nerve cord (VNC) of stage 15 embryos stained with BP102 (A–F) and shown with anterior up. (A) Control embryo heterozygous for elav-Gal4 and w1118. Thick longitudinal connectives connect each hemisegment (arrowhead). The arrow denotes the space between the anterior and posterior commissural axons. (B and C) After overexpression of jing (B) or DATR-X (C) in postmitotic CNS neurons, longitudinal axons (arrowheads) are very thin and the space between commissural axons is smaller (arrows). (D) Coexpression of one copy of EP(3)0635 and UAS-jingE in postmitotic neurons has synergistic effects. Note the absence of longitudinal connectives (arrowhead). (E and F) Additive effects of overexpressing two copies of UAS-jingE (E) or EP(3)0635 (F) Note that commissural axons are often not separated (arrows) and longitudinal connectives are very thin (arrowheads). (G) Quantitative analysis of longitudinal connective formation. Bars, 50 μm.
Pan-neural coexpression of jing and DATR-X resulted in more severe defects in axonal patterning than expression of one or two copies of either transgene alone. An average of 65% of segments had no longitudinal connectives after jing and DATR-X coexpression (Figure 5D, arrowhead; Figure 5G) in comparison with expression of two copies of UAS-jing (5% of segments, n = 80) and EP(3)0635 (18% of segments, n = 55) (Figure 5G). Therefore, jing and DATR-X coexpression has synergistic effects specifically in embryonic CNS neurons.
Neuronal-specific functions of jing and DATR-X are required for repulsion of longitudinal axons and glia from the CNS midline:
We targeted DATR-X and jing mutations to discern the neuronal and glial contributions of each gene product to axon scaffold formation. DATR-X and jing expression was knocked down using conditional RNAi (Hammond et al. 2001; Lee and Carthew 2003). A total of 697- and 567-bp sequences from nonconserved regions of DATR-X and jing were separately subcloned into a P[UAST] derivative plasmid to produce intron-spliced hairpin RNA corresponding to the DATR-X and jing genes, respectively. The UAS/Gal4 system (Brand and Perrimon 1993) was then used to allow hairpin RNA to conditionally downregulate DATR-X and jing expression in specific cell lineages, which was confirmed by in situ hybridization. Penetrance values of CNS axon phenotypes associated with neuronal and glial knockdown of jing and DATR-X ranged from 24 to 30% (see materials and methods).
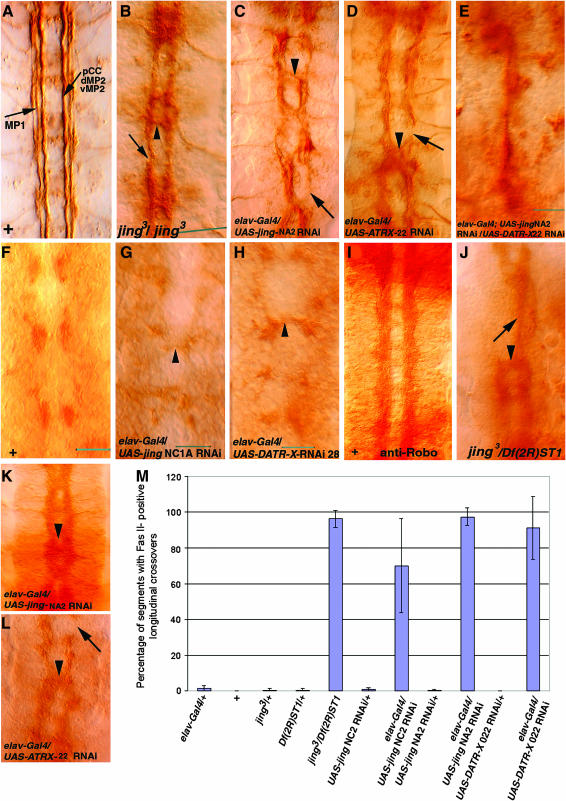
Longitudinal connective formation was analyzed in homozygous jing mutant embryos and those with neuronal-specific knockdown of jing and DATR-X stained with an antibody to Fasciclin II (1D4). Neuronal specificity was directed by the ELAV-Gal4 driver. In these mutant embryos, FasII-positive longitudinal axons aberrantly cross the CNS midline in stage 16 embryos (Figure 6, B–D, arrowheads; Figure 6M). In addition, there are breaks in the longitudinal connectives suggesting axon outgrowth defects (Figure 6, C and D, arrows). In embryos with simultaneous neuronal knockdown of jing and DATR-X all FasII-positive lateral fascicles fuse into a single tract at the CNS midline (Figure 6E). These results establish an autonomous neuronal requirement for jing and DATR-X function in the outgrowth and lateral positioning of longitudinal axons and a genetic synergy during this process.
Figure 6.—
DATR-X and jing functions are required specifically in CNS neurons for longitudinal connective formation. DATR-X and jing RNAi transgene expression was driven in neurons by ELAV-Gal4. (A–E and F–L) Shown are close-up views of the VNC of whole-mount stage16 embryos stained with anti-Fasciclin II monoclonal antibody (1D4) (A–E) and anti-Robo (F–L). Stage 12 embryos stained with anti-Robo (F–H). (A) In wild-type embryos, three longitudinal fascicles are clearly delineated. (B–D) In jing (B and C) and DATR-X (D) mutants, longitudinal fascicles misroute across the midline (arrowheads) and are broken (arrows). (E) After coexpression of jing and DATR-X RNAi transgenes in neurons, longitudinal fascicles fuse into one tract at the midline. (F) Wild-type Robo-positive glia are present on each side of the midline. (G and H) Medial misplacement of Robo-positive glia (arrowheads) occurs after neuronal-specific knockdown of jing and DATR-X. (I) Lateral positioning of Robo-positive axons in wild-type embryos. (J–L) Robo-positive axons cross the midline (arrowheads) after neuronal-specific knockdown of DATR-X and jing. Axonal breaks are evident (arrow in L). Arrow in J denotes remaining connective. (M) Graph displaying the average number of longitudinal axons that cross over the CNS midline per embryo.
Consistent with these results, pan-neural knockdown of jing or DATR-X was associated with high levels of Robo at the CNS midline during both stage 12 (Figure 6, G and H) and stage 16 (Figure 6, J and K). The phenotypes were variable (Figure 6K), as in the most severe case all Robo-positive axons were present at the midline (Figure 6K) and in less severe cases midline Robo was observed in fewer hemisegments (Figure 6L, arrowhead). We also observed breaks in Robo-positive connectives (Figure 6L, arrow). The high Robo levels in jing and DATR-X pan-neural mutants suggest that these genes do not regulate robo expression. However, the medial axon displacement suggests that these mutations may affect the expression of other genes required for Robo to read the Slit cue. Alternatively, jing and DATR-X may regulate longitudinal positioning in a Robo-independent manner (Kinrade et al., 2001).
Glial functions of Jing and DATR-X are required for ipsilateral positioning of longitudinal glia, glial survival, and patterning of longitudinal axons:
Genetic and cell ablation experiments have shown the critical role that neuronal–glial communication plays during pioneering of the longitudinal axon tracts (Booth et al. 2000; Hidalgo and Booth 2000; Hidalgo et al. 2001; Kinrade et al. 2001; Whitington et al. 2004). We therefore thought it might be informative to examine whether jing and DATR-X play a role in glial guidance of longitudinal axons. Glial development was examined after pan-glial expression of jing and DATR-X RNAi transgenes under the control of glial cells missing (gcm)-Gal4 (Hosoya et al. 1995; Jones et al. 1995) and using anti-Repo as a marker to assess glial fates (Campbell et al. 1994; Xiong et al. 1994; Halter et al. 1995).
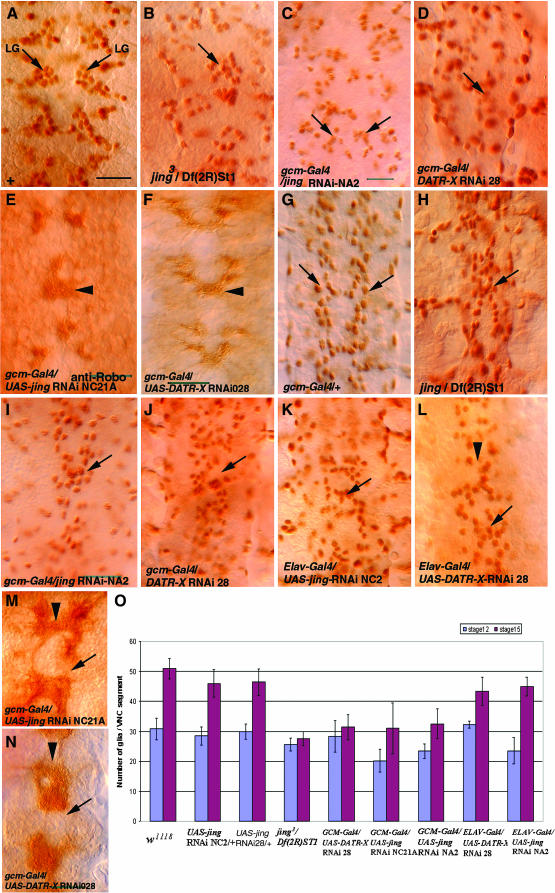
Robo-positive and Repo-positive LG were already misplaced medially during stage 12 after pan-glial knockdown of jing and DATR-X (Figure 7, C–F) and in jing hemizygotes (Figure 7B). Wild-type numbers of glia in these mutants at this stage reveal that jing and DATR-X are involved in glial differentiation and not in the division or specification of these cells (Figure 7O). Later during wild-type embryogenesis Robo is present only in axons (see Figure 6I) and therefore glial movement is restricted by Robo-independent mechanisms, including axon contact and trophic support (Kinrade et al., 2001). In the mature cord, we found that both LG and longitudinal axons were medially misplaced to the CNS midline in jing hemizygotes and after glial knockdown of jing and DATR-X (Figure 7, H–J, M, N). Despite the maintenance of glial–axonal contact, the numbers of total glia steadily decreased during embryogenesis in jing and DATR-X glial mutants (Figure 7O). However, glial numbers were unaffected after neuronal-specific jing and DATR-X knockdown despite a medial misplacement and mispositioning of LG (Figure 7, K, L, O). Therefore, jing and DATR-X glial-specific mutations perturb an autonomous survival function that cannot be compensated for by axon contact.
Figure 7.—
DATR-X and jing are required for glial patterning and survival. (A–D and G–L) CNS glia were identified in whole-mount embryos stained with anti-Repo. (E, F, M, and N) Embryos stained with anti-Robo. Shown are close-up ventral views of the nerve cord of stage 12/3 (A–F) and stage 15 (G–N) embryos. (A) In wild-type stage 12/3 embryos, longitudinal glia (LG) have already migrated medially to their positions to guide pioneer longitudinal axons. (B–D) In contrast, LG inappropriately occupy positions at the CNS midline in hemizygous jing embryos (B) and in those with glial-specific knockdown of jing (C) and DATR-X (D) as driven by gcm-Gal4. (E and F) Robo-positive glia are misplaced medially after expression of jing (E) and DATR-X (F) RNAi transgenes in CNS glia. (G) In wild-type embryos, the LG occupy a two-cell wide row lining the longitudinal connectives (arrows). The LG are observed only in the dorsal plane of view. (H–J) In jing hemizygotes (H) and in jing (I) and DATR-X (J), glial mutants glia are misplaced across the midline (arrows). (K and L) In jing (K) and DATR-X (L) neuronal mutants, glia cross the midline but are also scattered in the nerve cord. (M and N) Glial knockdown of jing (M) and DATR-X (N) results in breaks in Robo-positive longitudinal axons (arrows) and inappropriate midline crossings (arrowheads). (N) Quantitative analysis of glia during stages 12 and 15 after expressing jing and DATR-X RNAi transgenes in glia or neurons. Bars, 50 μm.
DISCUSSION
A conserved role for ATR-X in the embryonic CNS?
Of the candidates from the screen, we chose to further study DATR-X due to a possible involvement in Jing CNS function and disease relevance. Mutations in the human ATR-X gene are associated with several X-linked MR phenotypes that lead to cognitive delay, facial dysmorphism, microcephaly, skeletal and genital abnormalities, and neonatal hypotonia (Gibbons et al. 1995a,b; Villard et al. 1996a,b; Gibbons and Higgs 2000). A total of 87% of MR genes have a fruit fly homolog and 76% have a candidate functional ortholog revealing a remarkable conservation between humans and D. melanogaster (Inlow and Restifo 2004). Some orthologs of human MR genes have cellular phenotypes involving neurons, glia, and neural precursor cells and arise from defects in proliferation, migration, and process extension or arborization (Inlow and Restifo 2004). For example, targeted mutation of ATR-X to the early forebrain in mice leads to cortical progenitor cell death and reduced forebrain size (Bérubé et al. 2005). In addition, mutations in genes controlling the identity of forebrain neuronal precursors can result in holoprosencephaly in which the brain hemispheres do not separate (Wallis and Muenke 2000). An increased understanding of the molecular and cellular bases for hereditary MR is critical for the generation of drug treatments.
ATR-X belongs to the SWI/SNF group of chromatin remodeling proteins, which use the energy provided by ATP hydrolysis to disrupt histone–DNA associations and move nucleosomes to different positions (Kingston and Narlikar 1999; Whitehouse et al. 1999). This chromatin modulation allows for the access of activators or repressors to their DNA binding sites in their target genes. The helicase C and SNF2N domains of ATR-X have been shown to have DNA translocase and nucleosome-remodeling activities (Xue et al. 2003; Tang et al. 2004). Accordingly, mutations in ATR-X have been mapped to the helicase C and SNF2N domains, which show ∼60% homology with those in DATR-X and have been conserved from C. elegans to humans. This conservation supports a conserved role for Drosophila ATR-X in chromatin remodeling (Tang et al. 2004; this work).
Vertebrate ATR-X has a C2C2 zinc-finger motif in the amino terminus that is similar to a plant homeodomain finger previously identified in proteins involved in chromatin-mediated transcriptional regulation (Aasland et al. 1995; Gibbons et al. 1997). Interestingly, D. melanogaster and C. elegans ATR-X proteins do not contain the zinc-finger domain, suggesting that these structures may have been acquired through evolution due to a necessity in vertebrate chromatin remodeling mechanisms. Patients have been identified with mutations in the ATPase and zinc-finger domains of ATR-X, confirming that these are essential functional regions of the protein (Villard et al. 1996b,c; Gibbons and Higgs 2000).
Given the absence of the zinc-finger domains in DATR-X, we postulate that invertebrate DATR-X proteins may be complexed with proteins containing a nuclear targeting and DNA-binding motif to regulate gene expression at the proper regulatory sites. This may be one role of Jing since it has an embryonic expression pattern as well as mutant and overexpression phenotypes very similar to those of DATR-X. Therefore, it seems that the ATPase domain of DATR-X has been conserved through evolution and that the other regions of the protein may have evolved to suit the specific needs of the cell. In summary, different mechanisms of ATR-X function and different binding partners across species may account for the divergence of sequence with respect to the amino terminal and Q-rich repeats while the main chromatin remodeling aspects of ATR-X remain similar.
Jing encodes a nuclear protein with putative DNA-binding and transcriptional regulatory domains (Liu and Montell 2001; Sedaghat et al. 2002). The C2H2 zinc fingers of Jing are most similar (50% identical) to those of the mouse adipocyte enhancer binding protein 2 (AEBP2) and also show 25% homology to those of the Krüppel family of transcription factors, including those encoding gli and ZIC2 (Liu and Montell 2001). AEBP2 function is implicated in chromatin remodeling events (Cardoso et al. 1998; Cao and Zhang 2004) and has strong expression in the brain (He et al. 1999).
Genetic screening identifies a related group of jing-interacting genes:
We have utilized a background sensitive to jing function to conduct a genetic screen in the eye. For the GOF screen, we hypothesized that misexpression of jing in the eye in combination with other genes involved in jing transcriptional regulation would lead to alterations in gene expression and consequently disrupt ommatidial formation. The genetic relationship between DATR-X and jing in embryonic neurons and glia shows that the screen was successful in identifying genes whose function in adult neuronal cells is relevant to jing function in the embryonic CNS. This is consistent with previous findings that the fly eye is a valid system for targeting genes that function in other tissues (Raymond et al. 2004).
EP(3)3084 contains a transposon in proximity to a novel gene known by its FlyBase transcript identifier as CG15507. Despite strong effects of EP(3)3084 expression in the eye these were specifically strongly enhanced after coexpression with jing, DAtx2, and JIGR1. Furthermore, each gene specifically interacted with each other but not with randomly chosen EP lines, suggesting a functional relationship between the four genes. The EP elements in these lines are located in the 5′ untranslated region of the downstream genes, suggesting they may result in overexpression (BDGP). Given the regulatory role of MADF domains, it is possible that JIGR1 regulates gene expression with Jing and DATR-X (England et al. 1992; Bhaskar and Courey 2002). Alternatively, JIGR1 may regulate the expression of a Jing/DATR-X target gene. Likewise, DAtx2 may be involved in regulating the translation of a protein that is an essential component of a Jing/DATR-X/JIGR1 complex or a downstream target of these genes (Satterfield et al. 2002; Ciosk et al. 2004). A role for the orthologs of translational regulators in MR has been shown for the Drosophila ortholog of fragile-X MR 1 (Dfmr1). Dfmr1 regulates the MAP1B homolog of Futsch to control synaptic structure and function in the embryonic Drosophila CNS (Zhang et al. 2001). Therefore, genetic screening and phenotypic analysis in Drosophila have the power to decipher pathways and the cellular bases of MR genes.
Neuronal and glial functions of jing and DATR-X dictate axon tract formation:
In wild-type Drosophila embryos, LG assume characteristic positions and do not cross the midline or into adjacent VNC segments (Ito et al. 1995). This is due to multiple mechanisms at different stages of development, including response to repulsive and attractive molecules, cell–cell contact, trophic support, and axon contact (Kinrade et al., 2001). A disruption in any of these processes will perturb formation of the glial and axonal scaffolds. The expression of jing and DATR-X reporter genes in LG correlates with the LG phenotypes associated with mutations in these genes.
During stage 12, Robo present on the LG responds to repulsive midline Slit molecules to maintain lateral positioning (Kinrade et al. 2001). The medial misplacement of Robo- and Repo-positive LG during stage 12 after jing and DATR-X glial-specific knockdown suggests that there may have been a breakdown in Robo-dependent repulsive mechanisms. However, the fact that Robo protein was present after jing and DATR-X glial and neuronal knockdown suggests that robo expression may not be regulated by Jing and DATR-X. Alternatively, Jing and DATR-X may regulate the expression of a factor that controls how Robo reads the Slit signal. In support, misrouting of axons across the midline in the presence of Robo occurs in calmodulin and Son of sevenless mutants where these proteins are required to process the Sli signal (Fritz and VanBerkum 2000). It is also possible that jing and DATR-X regulate the expression of factors controlling glial and neuronal positioning in a Robo-independent fashion (Kinrade et al. 2001).
jing and DATR-X mutations clearly affect more than Robo-mediated LG positioning. First, glial survival is not affected in robo mutant embryos, whereas glia die despite continuous axonal contact in jing and DATR-X glial-specific mutants. Therefore, the loss of CNS glia may reflect a breakdown in an intrinsic survival pathway mediated by jing and DATR-X. The expression of jing and DATR-X reporter genes in glia is consistent with such a role. Furthermore, both jing and DATR-X/ATR-X have been implicated in cell survival processes in the CNS midline and tracheal cells and in cortical progenitors, respectively (Sedaghat et al. 2002; Sonnenfeld et al. 2004; Bérubé et al. 2005).
Second, in robo mutants only the central pCC/MP2 fascicle, but not the outer two longitudinal fascicles, is affected. However, in jing and DATR-X glial and neuronal mutants the outer fascicles are fused, often broken, and can be seen crossing the midline. These defects are similar to those after ablation of neurons or glia and after genetic loss of glia as in gcm mutants (Hosoya et al. 1995; Jones et al. 1995; Vincent et al. 1996; Hidalgo and Brand 1997). These observations suggest that multiple biological processes require the proper function of these genes and are consistent with an important upstream role for jing and DATR-X in glial and neuronal differentiation.
Evidence is accumulating that chromatin accessibility plays a key role in the transcriptional regulation of cell-type-specific gene expression in the CNS (Hsieh et al. 2004). The conservation in ATPase domains along with the similar phenotype of DATR-X and jing mutations and in their expression patterns raises the possibility that Jing is involved in the targeting of a chromatin remodeling complex containing DATR-X to transcriptional target genes whose products are required for the response of longitudinal growth cones and glia to guidance cues.
In summary, we have identified a group of genes that pertain to jing function and specifically genetically interact in adult neuronal cells. Our results show that specific neural and glial developmental defects underlie the problems in axon guidance associated with mutations in DATR-X and jing. More studies using targeted mutations of MR genes will alleviate the view that brain phenotypes result from generic effects due to a heightened sensitivity of the brain.
Acknowledgments
We thank Marc Freeman for gcm-gal4 and S. Hayashi for btl-gal4. We thank Doug Holmyard at the Bioimaging Centre at Mount Sinai Hospital for performing SEM. We thank Helga Agah for help with immunohistochemistry. We thank Andrew Ridsdale and the OGHRI for use of the confocal microscope and training. This work was supported by a grant from the Canadian Institutes of Health Research to M.S.
References
- Aasland, R., T. J. Gibson and A. F. Stewart, 1995. The PHD finger: implications for chromatin-mediated transcriptional regulation. TIBS 20: 56–59. [DOI] [PubMed] [Google Scholar]
- Abidi, F., N. J. Carpenter, L. Villard, M. Curtis, M. Fontes et al., 1999. The Carpenter-Waziri syndrome results from a mutation in DATR-X. Am. J. Med. Genet. 85: 249–251. [DOI] [PubMed] [Google Scholar]
- Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne et al., 2000. The genome sequence of Drosophila melanogaster. Science 5461: 2185–2195. [DOI] [PubMed] [Google Scholar]
- Ahmad, K., and S. Henikoff, 2001. Modulation of a transcription factor counteracts heterochromatic gene silencing in Drosophila. Cell 104: 839–847. [DOI] [PubMed] [Google Scholar]
- Aravind, L., 2000. The BED finger, a novel DNA-binding domain in chromatin- boundary-element-binding proteins and transposases. Trends Biochem. Sci. 25: 421–423. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Ashley, C. T., C. G. Pendleton, W. W. Jennings, A. Saxena and C. V. Glover, 1989. Isolation and sequencing of cDNA clones encoding Drosophila chromosomal protein D1. A repeating motif in proteins which recognize at DNA. J. Biol. Chem. 264: 8394–8401. [PubMed] [Google Scholar]
- Bate, C. M., and E. B. Grunewald, 1981. Embryogenesis of an insect nervous system II: a second class of neuron precursor cells and the origin of the intersegmental connectives. J. Embryol. Exp. Morphol. 61: 317–330. [PubMed] [Google Scholar]
- Barresi, M. J. F., L. D. Hutson, C-B. Chien and R. O. Karlstrom, 2005. Hedgehog regulated Slit expression determines commissure and glial cell position in the zebrafish forebrain. Development 132: 3643–3656. [DOI] [PubMed] [Google Scholar]
- Battye, R., A. Stevens and J. R. Jacobs, 1999. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development 126: 2475–2481. [DOI] [PubMed] [Google Scholar]
- Bérubé, N. G., M. Mangelsdorf, M. Jagla, J. Vanderluit, D. Garrick et al., 2005. The chromatin-remodeling protein ATRX is critical for neuronal survival during corticogenesis. J. Clin. Invest. 115: 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, V., and A. J. Courey, 2002. The MADF-BESS domain factor Dip3 potentiates synergistic activation by Dorsal and Twist. Gene 299: 173–184. [DOI] [PubMed] [Google Scholar]
- Bhat, K. M., 2005. Slit-Roundabout signaling neutralizes netrin-frazzled-mediated attractant cue to specify the lateral positioning of longitudinal axon pathways. Genetics 170: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, G. E., E. F. Kinrade and A. Hidalgo, 2000. Glia maintain follower neuron survival during Drosophila CNS development. Development 127: 237–244. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Brody, T., C. Stivers, J. Nagle and W. F. Odenwald, 2002. Identification of novel Drosophila neural precursor genes using a differential embryonic head cDNA screen. Mech. Dev. 113: 41–59. [DOI] [PubMed] [Google Scholar]
- Campbell, G., H. Goring, T. Lin, E. Spana, S. Anderson et al., 1994. RK2, a glila-specific homeodomain protein required for embryonic nerve cord condensation and viability in Drosophila. Development 120: 2957–2966. [DOI] [PubMed] [Google Scholar]
- Cao, R., and Y. Zhang, 2004. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol. Cell 15(1): 57–67. [DOI] [PubMed] [Google Scholar]
- Cardoso, C., S. Timsit, L. Villard, M. Khrestchatisky, M. Fontes et al., 1998. Specific interaction between the ATR-X gene product and the SET domain of the human EZH2 protein. Hum. Mol. Genet. 7: 679–684. [DOI] [PubMed] [Google Scholar]
- Cardoso, C., Y. Lutz, C. Mignon, E. Compe, D. Depetris et al., 2000. ATR-X mutations cause impaired nuclear location and altered DNA binding properties of the ATR-X protein. J. Med. Genet. 37: 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosk, R., M. DePalma and J. R. Priess, 2004. ATX-2, the C. elegans ortholog of ataxin 2, functions in translational regulation in the germline. Development 131: 4831–4841. [DOI] [PubMed] [Google Scholar]
- Cutler, G., K. M. Perry and R. Tjian, 1998. Adf-1 is a nonmodular transcription factor that contains a TAF-binding Myb-like motif. Mol. Cell Biol. 18: 2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn, R., Jr., and S. Kunes, 2004. An axon scaffold induced by retinal axons directs glia to destinations in the Drosophila optic lobe. Development 131: 2291–2303. [DOI] [PubMed] [Google Scholar]
- England, B. P., A. Admon and R. Tjian, 1992. Cloning of the Drosophila transcription factor Adf-1 reveals homology to Myb oncoproteins. Proc. Natl. Acad. Sci. USA 89: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewel, A., J. R. Jackson and C. Benyajati, 1990. Alternative DNA-protein interactions in variable-length internucleosomal regions associated with Drosophila Adh distal promoter expression. Nucleic Acids Res. 18: 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M. R., J. Delrow, J. Kim, E. Johnson and C. Q. Doe, 2003. Unwrapping glial biology: Gcm target genes regulating glial development, diversification and function. Neuron 38: 567–580. [DOI] [PubMed] [Google Scholar]
- Fritz, J. L., and M. F. VanBerkum, 2000. Calmodulin and son of sevenless dependent signaling pathways regulate midline crossing of axons in the Drosophila CNS. Development 27(9): 1991–2000. [DOI] [PubMed] [Google Scholar]
- Gibbons, R. J., and D. R. Higgs, 2000. Molecular-clinical spectrum of the ATR-X syndrome. Am. J. Med. Genet. 97: 204–212. [DOI] [PubMed] [Google Scholar]
- Gibbons, R. J., L. Brueton, V. J. J. Buckle Burn, J. Clayton-Smith, B. C. C. Davison et al., 1995. a The clinical and hematological features of the X-linked α-thalassemia/mental retardation syndrome (ATR-X). Am. J. Med. Genet. 55: 288–299. [DOI] [PubMed] [Google Scholar]
- Gibbons, R. J., D. J. Picketts, L. Villard and D. R. Higgs, 1995. b Mutations in a putative global transcriptional regulator cause X-linked mental retardation with α-thalassemia (ATR-X syndrome). Cell 80: 837–845. [DOI] [PubMed] [Google Scholar]
- Gibbons, R. J., S. Bachoo, D. J. Picketts, S. Aftimos, B. Asenbauer et al., 1997. Mutations in transcriptional regulator ATRX establish the functional significance of a PHD-like domain. Nat. Genet. 17: 146–148. [DOI] [PubMed] [Google Scholar]
- Halter, D. A., J. Urban, C. Rickert, S. S. Ner, K. Ito et al., 1995. The homeobox gene repo is required for the differentiation and maintenance of glia function in the embryonic nervous system of Drosophila. Development 121: 317–332. [DOI] [PubMed] [Google Scholar]
- Hammond, S. M., A. A. Caudy and G. J. Hannon, 2001. Post-transcriptional gene silencing by double-stranded RNA. Nat. Rev. Genet. 2(2): 110–119. [DOI] [PubMed] [Google Scholar]
- Hay, B. A., T. Wolff and G.M. Rubin, 1994. Expression of baculovirus P35 prevents cell death in Drosophila. Development 120: 2121–2129. [DOI] [PubMed] [Google Scholar]
- He, G.-P., S. Kim and H.-S. Ro, 1999. Cloning and characterization of a novel zinc finger transcriptional repressor. J. Biol. Chem. 274: 14678–14684. [DOI] [PubMed] [Google Scholar]
- Hidalgo, A., and A. H. Brand, 1997. Targeted neuronal ablation: the role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development 124: 3253–3262. [DOI] [PubMed] [Google Scholar]
- Hidalgo, A., and G. Booth, 2000. Glia dictate trajectories of pioneer neurons in the Drosophila embryonic CNS. Development 127: 393–402. [DOI] [PubMed] [Google Scholar]
- Hidalgo, A., E. F Kinrade and M. Georgiou, 2001. The Drosophila neuregulin vein maintains glial survival during axon guidance in the CNS. Dev. Cell 1: 679–690. [DOI] [PubMed] [Google Scholar]
- Hosoya, T., K. Takizawa, K. Nitta and Y. Hotta, 1995. Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82: 1025–1036. [DOI] [PubMed] [Google Scholar]
- Hsieh, J., K. Nakashima, T. Kuwabara, E. Mejia and F. H. Gage, 2004. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 101: 16659–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., S. J. Myers and R. Dingledine, 1999. Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat. Neurosci. 2(10): 867–872. [DOI] [PubMed] [Google Scholar]
- Hulo, N., C. J. A. Sigrist, V. Le Saux, P. S. Langendijk-Genevaux, L. Bordoli et al., 2004. Recent improvements to the PROSITE database. Nucleic Acids Res. 32: D134–D137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inlow, J. K., and L. L. Restifo, 2004. Molecular and comparative genetics of mental retardation. Genetics 166: 835–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, K., J. Urban and G. M. Technau, 1995. Distribution, classification and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux's Arch. Dev. Biol. 204: 284–307. [DOI] [PubMed] [Google Scholar]
- Jacobs, J. R., and C. S. Goodman, 1989. Embryonic development of axon pathways in the Drosophila CNS. II. Behavior of pioneer growth cones. J. Neurosci. 9: 2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janody, F., J. Reischl and N. Dostatni, 2000. Persistence of Hunchback in the terminal region of the Drosophila blastoderm embryo impairs anterior development. Dev. 127: 1573–1582. [DOI] [PubMed] [Google Scholar]
- Jones, B. W., R. D. Fetter, G. Tear and C. S. Goodman, 1995. Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Keleman, K., S. Rajagopalan, D. Cleppien, D. Teis, K. Palha et al., 2002. Comm sorts Robo to control axon guidance at the Drosophila midline. Cell 110: 415–427. [DOI] [PubMed] [Google Scholar]
- Keleman, K., C. Ribeiro and B. J. Dickson, 2005. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat. Neurosci. 8: 156–163. [DOI] [PubMed] [Google Scholar]
- Kidd, T., K. Brose, K. J. Mitchell, R. D. Fetter, M. Tessier-Lavigne et al., 1998. a Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionary conserved guidance receptors. Cell 92: 205–215. [DOI] [PubMed] [Google Scholar]
- Kidd, T., C. Russell, C. S. Goodman and G. Tear, 1998. b Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron 20: 25–33. [DOI] [PubMed] [Google Scholar]
- Kidd, T., K. S. Bland and C. S. Goodman, 1999. Slit is the midline repellent for the Robo receptor in Drosophila. Cell 96: 785–794. [DOI] [PubMed] [Google Scholar]
- Kingston, R. E., and G. J. Narlikar, 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13: 2339–2352. [DOI] [PubMed] [Google Scholar]
- Kinrade, E. F. V., T. Brates, G. Tear, and A. Hidalgo, 2001. Roundabout signaling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development 128: 207–216. [DOI] [PubMed] [Google Scholar]
- Lasko, P., 2000. The Drosophila melanogaster genome: translation factors and RNA binding proteins. J. Cell Biol. 150: F51–F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. S., and R. W. Carthew, 2003. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30: 322–329. [DOI] [PubMed] [Google Scholar]
- Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks et al., 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32: D142–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., and D. J. Montell, 2001. jing: a downstream target of slbo required for developmental control of border cell migration. Development 128: 321–330. [DOI] [PubMed] [Google Scholar]
- Long, H., C. Sabatier, L. Ma, A. Plump, W. Yuan et al., 2004. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42: 213–223. [DOI] [PubMed] [Google Scholar]
- Oland, L. A., and L. P. Tolbert, 2002. Key interactions between neurons and glial cells during neural development in insects. Annu. Rev. Entomol. 48: 89–110. [DOI] [PubMed] [Google Scholar]
- Page-McCaw, P. S., K. Amonlirdviman and P. A. Sharp, 1999. PUF60: a novel U2AF65-related splicing activity. RNA 5: 1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Rangel, M. T., I. Rodriguez and J. R. Riesgo-Escovar, 2002. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics 160: 1035–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picketts, D. J., D. R. Higgs, S. Bachoo, D. J. Blake, O. W. J. Quarrell et al., 1996. ATRX encodes a novel member of the SNF2 family of proteins: mutations point to a common mechanism underlying the ATR-X syndrome. Hum. Mol. Genet. 5: 1899–1907. [DOI] [PubMed] [Google Scholar]
- Rajagopalan, S., E. Nicolas, V. Vivancos, J. Berger and B. J. Dickson, 2000. a Crossing the midline. Roles and regulation of Robo receptors. Neuron 28: 767–777. [DOI] [PubMed] [Google Scholar]
- Rajagopalan, S., V. Vivancos, E. Nicolas and B. J. Dickson, 2000. b Selecting a longitudinal pathway. Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell 103: 1033–1045. [DOI] [PubMed] [Google Scholar]
- Rasband, K., M. Hardy and C. B. Chien, 2003. Generating X: formation of the optic chiasm. Neuron 39: 885–888. [DOI] [PubMed] [Google Scholar]
- Raymond, K., E. Bergeret, A. Avet-Rochex, R. Griffin-Shea and M.-O. Fauvarque, 2004. A screen for modifiers of RacGAP(84C) gain-of-function in the Drosophila eye revealed the LIM kinase Cdi/TESK1 as a downstream effector of Rac1 during spermatogenesis. J. Cell Sci. 117: 2777–2789. [DOI] [PubMed] [Google Scholar]
- Robinow, S., and K. White, 1988. The locus ELAV of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev. Biol. 126: 294–303. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Alfageme, G. T., G. T. Rudkin and L. Cohen, 1980. Isolation, properties and cellular distribution of D1, a chromosomal protein of Drosophila. Chromosoma 78: 1–31. [DOI] [PubMed] [Google Scholar]
- Rørth, P., 1996. A modular misexpression screen in Drosophila detecting tissue- specific phenotypes. Proc. Natl. Acad. Sci. USA 93: 12418–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., 1988. Drosophila melanogaster as an experimental organism. Science 240: 1453–1459. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1983. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 11: 6341–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., L. Hong, P. Brokstein, M. Evans-Holm, E. Frise et al., 2000. A Drosophila complementary DNA resource. Science 5461: 2222–2224. [DOI] [PubMed] [Google Scholar]
- Satterfield, T. F., S. M. Jackson and L. J. Pallanck, 2002. A Drosophila homolog of the polyglutamine disease gene SCA2 is a dosage-sensitive regulator of actin filament formation. Genetics 162: 1687–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat, Y., W. Miranda and M. Sonnenfeld, 2002. The jing Zn finger transcription factor is a mediator of cellular differentiation in the Drosophila CNS midline and trachea. Development 129: 2591–2606. [DOI] [PubMed] [Google Scholar]
- Seeger, M., G. Tear, D. Ferres-Marco and C. S. Goodman, 1993. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron 10: 409–426. [DOI] [PubMed] [Google Scholar]
- Shiga, Y., M. Tanada-Matakatsu and S. A. Hayashi, 1996. Nuclear β-galactosidase fusion protein as a marker of morphogenesis in living Drosophila. Dev. Growth Differ. 38: 99–106. [Google Scholar]
- Simpson, J. H., K. S. Bland, R. D. Fetter and C. S. Goodman, 2000. a Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell 103(7): 1019–1032. [DOI] [PubMed] [Google Scholar]
- Simpson, J. H., T. Kidd, K. S. Bland and C. S. Goodman, 2000. b Short-range and long-range guidance by Slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron 28: 753–766. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld, M. J., N. Barazesh, Y. Sedaghat and C. Fan, 2004. The jing and ras1 pathways are functionally related during CNS midline and tracheal development. Mech. Dev. 121: 1531–1547. [DOI] [PubMed] [Google Scholar]
- Tang, J., S. Wu, H. Liu, R. Stratt, O. G. Barak et al., 2004. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279: 20369–20377. [DOI] [PubMed] [Google Scholar]
- Tatusova, T. A., and T. L. Madden, 1999. Blast 2 sequences-a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174: 247–250. [DOI] [PubMed] [Google Scholar]
- Tear, G., R. Harris, S. Sutaria, K. Kilomanski, C. S. Goodman et al., 1996. Commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron 16: 501–514. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., 1994. Axon guidance by diffusible repellants and attractants. Curr. Opin. Genet. Dev. 4: 596–601. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and C. S. Goodman, 1996. The molecular biology of axon guidance. Science 274: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Tseng, A. S., and I. K. Hariharan, 2002. An overexpression screen in Drosophila for genes that restrict growth or cell-cycle progression in the developing eye. Genetics 162: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanVactor, D., H. Sink, D. Fambrough, R. Tsoo and C. S. Goodman, 1993. Genes that control neuromuscular specificity in Drosophila. Cell 73: 1137–1153. [DOI] [PubMed] [Google Scholar]
- Villard, L., A. Toutain, A. M. Lossi, J. Gecz, C. Houdayer et al., 1996. a Splicing mutation in the ATR-X gene can lead to a dysmorphic mental retardation phenotype without α-thalassemia. Am. J. Hum. Genet. 58: 499–505. [PMC free article] [PubMed] [Google Scholar]
- Villard, L., P. Saugier-Veber, J. Gecz, J. F. Mattei, A. Munnich et al., 1996. b ATR-X mutation in a large family with Juberg-Marsidi syndrome. Nat. Genet. 12: 359–360. [DOI] [PubMed] [Google Scholar]
- Villard, L., J. Gecz, J. F. Mattei, M. Fontes, P. Saugler-Veber et al., 1996. c ATR-X mutation in a large family with Juberg-Marsidi syndrome. Nat. Genet. 4: 316–320. [DOI] [PubMed] [Google Scholar]
- Villard, L., M. Fontès and J. J. Ewbank, 1999. Characteriztion of ATR-X-1, a Caenorhabditis elegans gene similar to the human ATR-X gene. Gene 236: 13–19. [DOI] [PubMed] [Google Scholar]
- Vincent, S., J. L. Vonesch and A. Giangrande, 1996. Glide directs glial fate commitment and cell fate switch between neurons and glia. Development 122: 131–139. [DOI] [PubMed] [Google Scholar]
- Wallis, D., and M. Muenke, 2000. Mutations in holoprosencephaly. Hum. Mutat. 16(2): 99–108. [DOI] [PubMed] [Google Scholar]
- Whitehouse, I., A. Flaus, B. R. Cairns, M. F. White, J. L. Workman et al., 1999. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature 400: 784–787. [DOI] [PubMed] [Google Scholar]
- Whitington, P. M., C. Quilkey and H. Sink, 2004. Necessity and redundancy of guidepost cells in the embryonic Drosophila CNS. Int. J. Dev. Neurosci. 22: 157–163. [DOI] [PubMed] [Google Scholar]
- Wing, J. P., B. A. Schreader, T. Yokokura, Y. Wang, P. S. Andrews et al., 2002. Drosophila Morgue is an F box/ubiquitin conjugase domain protein important for grim-reaper mediated apoptosis. Nat. Cell Biol. 6: 451–456. [DOI] [PubMed] [Google Scholar]
- Xiong, W. C., H. Okano, N. H. Patel, J. A. Blendy and C. Montell, 1994. Repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 8: 981–994. [DOI] [PubMed] [Google Scholar]
- Xue, Y., R. Gibbons, Z. Yan, D. Yang, T. L. McDowell et al., 2003. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100: 10635–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, K.-M., and K. White, 1994. Neural specificity of ELAV expression: defining a Drosophila promoter for directing expression to the nervous system. J. Neurochem. 63: 41–51. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Q., A. M. Bailey, H. J. Matthies, R. B. Renden, M. A. Smith et al., 2001. Drosophila fragile X-related gene relates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107: 591–603. [DOI] [PubMed] [Google Scholar]
- Zipursky, S. L., and G. M. Rubin, 1994. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu. Rev. Neurosci. 17: 373–397. [DOI] [PubMed] [Google Scholar]