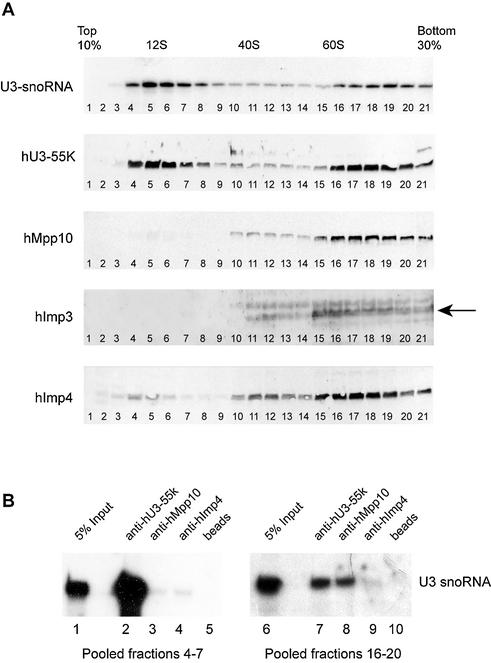
Figure 4.
hImp3, hImp4 and hMpp10 predominantly sediment at 60–80S in glycerol gradients. (A) HEp-2 cell lysates were loaded on a 10–30% (v/v) glycerol gradient, and the sedimentation of U3 snoRNA, hU3-55K, hMpp10, hImp3 and hImp4 was analyzed by northern blot hybridization and immunoblotting, respectively. The sedimentation of the large ribosomal RNAs was determined by agarose gel electrophoresis and ethidium bromide staining and these were used as markers for 40S and 60S particles in the gradient. The U1 snRNA was used as a marker for 12S complexes (data not shown). The arrow marks the hImp3 band. (B) The fractions containing either the 12S or 60–80S U3 snoRNP complexes (4–7 and 16–20) were pooled and used for immunoprecipitation experiments with anti-hU3-55K (lanes 2 and 7), anti-hMpp10 (lanes 3 and 8) and anti-hImp4 (lanes 4 and 9) antibodies. RNAs isolated from the pooled fractions (5% input, lanes 1 and 6) and co-precipitated RNAs were separated on denaturing polyacrylamide gels and analyzed by northern blot hybridization using a U3 snoRNA specific probe. As a negative control an immunoprecipitation was performed in the absence of antibodies (beads, lanes 5 and 10).