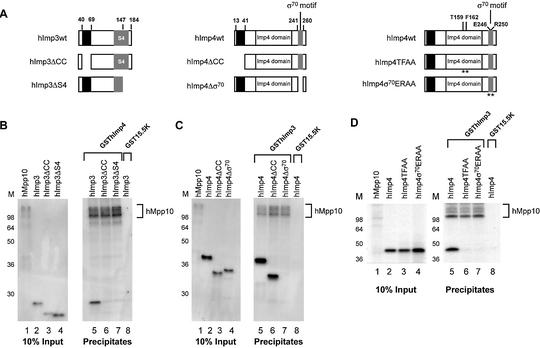
Figure 7.
Regions of hImp3 and hImp4 required for their interaction with hMpp10 in vitro. (A) Schematic representation of the hImp3 and hImp4 deletion and substitution mutants. The black boxes indicate the putative coiled-coil regions in hImp3 and hImp4. The putative RNA binding domains in hImp3 (S4) and hImp4 (σ70-like) are depicted as gray boxes. The Imp4 domain is marked with a white box. The asterisks indicate the positions in hImp4 that were changed in the substitution mutants. (B–D) Binding of hImp3 (B) and hImp4 (C) deletion mutants and hImp4 substitution mutants (D) to hMpp10. Reconstituted complexes were precipitated using glutathione–Sepharose beads as described in Materials and Methods and analyzed by SDS–PAGE. The hImp3 and hImp4 (mutant) translates are indicated above the respective lanes and radiolabeled hMpp10 was added to all reconstitution reactions. The GST- fusion proteins used in this experiment are indicated on top of the panels. Lanes 1–4 show 10% of the input material used in the pull-down experiments. Lanes 5–8 show the precipitates (in lane 8 GST15.5K was used as a negative control). On the left of each panel the positions of molecular mass markers are shown. On the right the position of hMpp10 in the gel is indicated.