Abstract
Giant-cell myocarditis is a rare and aggressive form of myocarditis with a high mortality rate. Our purpose is to summarize 3 cases of acute giant-cell myocarditis that illustrate possible outcomes with mechanical support.
We reviewed the cases of 3 patients, aged 39 to 59 years, who had giant-cell myocarditis (confirmed by myocardial biopsy). The indication for ventricular assist was circulatory failure despite maximal medical treatment with 2 or more inotropic agents and intraaortic balloon pump support. Immunosuppression and a biventricular mechanical assist (BVS 5000) were used to treat all these patients. The mean duration of mechanical support was 15.7 days (range, 10 to 19 days).
One patient had recovery of myocardial function and was weaned from mechanical support. This case is, to our knowledge, the first reported of ventricular support leading to cardiac recovery after diagnosis of giant-cell myocarditis. The 2nd patient was not a candidate for further surgery and died of multisystem organ failure. The 3rd patient underwent orthotopic heart transplantation after 18 days of support and was discharged.
We conclude that patients with giant-cell myocarditis tend to have biventricular involvement and can recover myocardial function on mechanical support and immunosuppression. If recovery is not observed, transplantation is warranted. By avoiding left ventricular cannulation, the BVS 5000 is well suited for bridging to recovery, transplantation, or long-term support. (Tex Heart Inst J 2003;30:50–6)
Key words: Autoimmune diseases, giant cells, heart-assist devices, heart transplantation, myocarditis
Giant-cell myocarditis (GCM) is a rare and aggressive form of myocarditis usually characterized by progressive congestive heart failure. The disease is seen predominantly in otherwise healthy, middle-aged patients and is treated initially with immunosuppression. In the absence of recovery of myocardial function, heart transplantation is the preferred method of treatment. However, cases of recurrence in the transplanted heart have been reported. 1,2 Effective use of a ventricular assist device can provide support during the period of potential recovery and assessment of transplant candidacy. Our purpose is to document our experience with temporary mechanical assistance in patients with rapidly progressive GCM, and to report a case of recovery.
BVS 5000 Assist Device
The BVS® 5000 Bi-ventricular Support System (Abiomed Corp.; Danvers, Mass) (Fig. 1) has been previously described. 3,4 Briefly, this device is a pneumatically driven, external, pulsatile pump consisting of a polyurethane chamber in a polycarbonate housing. 5,6 The atrium of the pump, which is separated from the ventricle by a 1-way valve that ensures unidirectional flow (Fig. 2), fills by gravity and empties passively following contraction of the ventricle. Upon the filling of the ventricle, the air pressure is sensed by the console, which immediately sends compressed air back to the pumping chamber, causing the bladder to eject its volume.
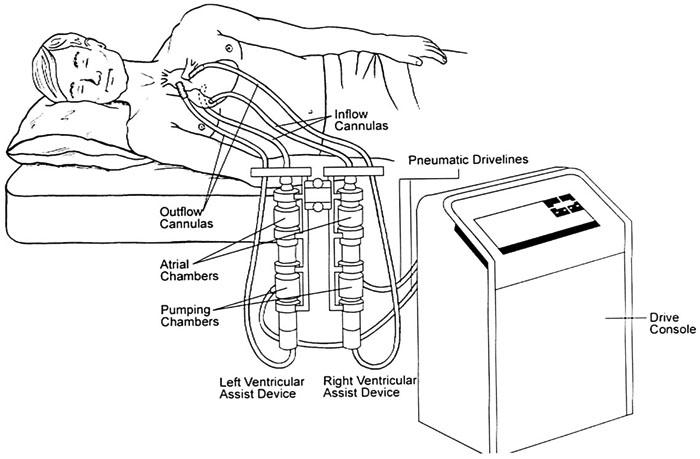
Fig. 1 The Abiomed BVS 5000 Bi-ventricular Support System. This rapidly deployable external device can be used for univentricular or biventricular support, depending on diagnosis and hemodynamics. It is designed for short-term use.

Fig. 2 How the BVS 5000 works: Note the passive filling, the two 1-way valves, and how the pneumatic drive is triggered by 80 cc of air displaced through the exhaust during pump diastole.
Reprinted from Ann Thorac Surg 1995;59(2 Suppl):S31–8, with permission from the Society of Thoracic Surgeons.
Implantation. The BVS 5000 is implanted via sternotomy. Left ventricular assist is achieved using a cannula with a graft extension sewn onto the aorta. Heparin is given to achieve an activated clotting time (ACT) of 300 seconds if the procedure is to be done off cardiopulmonary bypass (CPB). The ascending aorta is dissected at its distal portion, and a side-biting clamp is applied. An aortic graft is sewn on with an interrupted running 4-0 polypropylene suture reinforced with a strip of pericardium. In most cases, the left atrial cannula is inserted into the right superior pulmonary vein. The cannulae are positioned away from the heart and tunneled through the abdominal wall. The cannulae are then de-aired and connected to the BVS 5000 unit. Once left ventricular assist is started and the flows are initiated, the decision to start right ventricular assist is made. This implantation is carried out in the same manner; the pulmonary artery and the right atrium are used. A target flow of 5.5 L/min can usually be achieved. Activated clotting time (ACT) is targeted at 180 seconds. The device rate is set by cyclic filling, and self-adjustments are made to maintain a stroke volume of 80 cc. Hemodynamic instability during implantation dictates the use of CPB.
Removal. The midline sternotomy is reopened. Transesophageal echocardiography is used to assess cardiac function. Heparin is given to raise ACT to 250 seconds, and the VAD (ventricular assist device) flows are decreased to 2 L/min for 30 minutes. If hemodynamic function and echocardiographic observations are satisfactory, the cannulae are clamped. The outflow graft is then cut and the atrial cannula is removed. Pursestring sutures, which have been positioned in advance, are tied down. The stump of the outflow graft is oversewn. The sternal wound is copiously irrigated with antibiotic solution, followed by closure of the chest.
Case Reports
Patient 1
A 41-year-old man (175 cm, 56 kg) experienced chest pain and fatigue, followed by a syncopal episode and dyspnea, 4 days before transfer to our institution. At the outset, he had been treated with nonsteroidal anti-inflammatory agents. His echocardiogram was abnormal, with an ejection fraction of 0.35 and findings of concentric left ventricular hypertrophy.
Due to congestive heart failure, the patient was transferred to our institution. Upon his arrival, inotropic support was initiated and increased to maximal dosage. An intraaortic balloon pump (IABP) was inserted. Within the next 48 hours, the patient's left ventricular ejection fraction (LVEF) fell to 0.10–0.15. During an endomyocardial biopsy—which eventually confirmed giant cell myocarditis (Figs. 3A & B)—ventricular tachycardia developed. It was therefore decided to urgently implant the BVS 5000 for a presumed diagnosis of viral myocarditis.
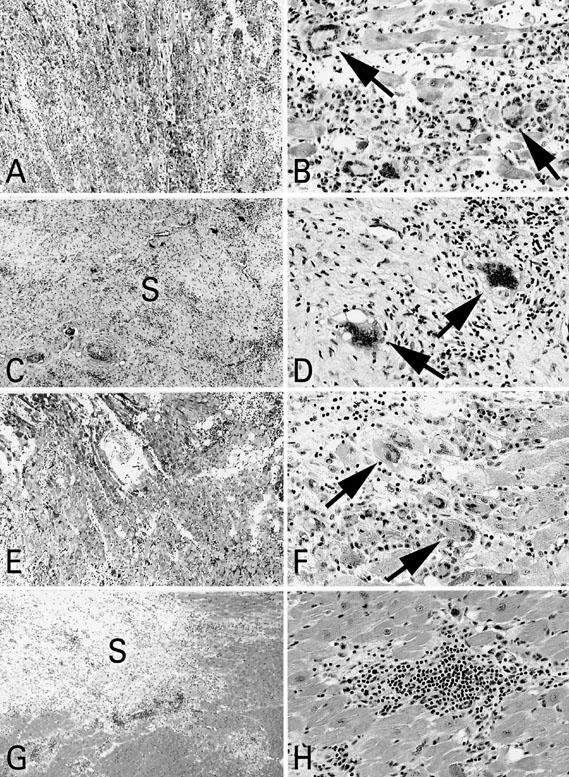
Fig. 3 Patient 1: A) Note diffuse mononuclear-cell infiltration of myocardium; B) Numerous multinucleated giant cells are present (arrows). Patient 2: C) Endomyocardial biopsy shows diffuse mononuclear cell infiltration and fibrous scar (S) tissue in myocardium; D) Occasional giant cells are present (arrows). Patient 3: E) Endomyocardial biopsy with extensive mononuclear cell infiltration; F) Numerous giant cells are present (arrows); G) Section of explanted heart shows mononuclear cell infiltrates and extensive fibrous scar (S) tissue proliferation; H) No giant cells were observed in association with the mononuclear-cell infiltrates.
After implantation of the BVS 5000's left ventricular assist device (LVAD), an attempt was made to wean the patient from cardiopulmonary bypass, but failure of the right ventricle occurred, despite infusion of epinephrine and milrinone. It was therefore decided to implant the right ventricular assist device (RVAD) of the BVS 5000. The IABP was removed. Postoperatively, the patient had persistent ventricular arrhythmias, which resolved with intravenous amiodarone.
Five days after the initiation of mechanical support, pulmonary artery catheter tracing showed right ventricular function to have recovered; the RVAD was therefore removed. The left ventricle was shown to have improved and was capable of ejection, but the ejection fraction was only 0.15–0.20. Five days after removal of the RVAD, and 10 days after initiation of all mechanical support, the left ventricle was seen to have recovered further, with an ejection fraction of 0.35 on echocardiography, upon volume loading. The LVAD was removed. During recovery, the patient experienced several episodes of ventricular tachycardia, which were confirmed to be inducible by a low-level stress test. Therefore, a cardiac defibrillator was implanted. His myocarditis was treated with cyclophosphamide (1 year) and prednisone (6 months). Current follow-ups at more than 3 years show the patient's condition to have completely recovered.
Patient 2
A 39-year-old man (180 cm, 96 kg) had been experiencing progressive symptoms thought to be related to upper respiratory tract infection, including chest pain, fatigue, and dyspnea, for a period of 3 months before being transferred to our hospital. These symptoms had been treated with antihistamines, and the patient's condition had improved until 2 weeks before transfer to our institution, when he experienced bradycardia and a seizure. He was found to be in complete heart block, and a DDD pacemaker was implanted. An echocardiogram showed an LVEF of 0.26, along with atrial dilation. Further instability necessitated support with inotropic agents and insertion of an IABP. After a myocardial biopsy confirmed GCM (Figs. 3C & D), the patient was transferred to our institution for further treatment. He had experienced right leg numbness 24 hours before being admitted.
By the time of his arrival, inotropic support had been increased to maximal doses of 3 different agents. The patient's low output persisted. He then underwent implantation of the BVS 5000 left ventricular assist device. During the procedure, the patient's condition became unstable and use of the heart-lung machine was required. The IABP was removed by means of the open technique. As the patient was weaned from cardiopulmonary bypass, it was noted that his right ventricle was failing, which required insertion of the BVS 5000 right ventricular assist device. Severe chest wall edema and renal failure made it very difficult to ventilate the patient. This required that his sternal wound be left open for several days postoperatively.
Upon completion of the implantation, the patient was noted to have an ischemic right foot. A vascular surgeon was consulted and urgent thrombectomy and fasciotomy were carried out. Although his condition initially improved, progressive vasodilation was observed. Worsening liver and renal function ensued. Because the patient's sepsis appeared to arise from his lower extremity, amputation was performed above the knee. Following this surgery, the right and left ventricular ejection fractions did not improve enough to enable removal of the device 10 days after admission. Further deterioration of liver, renal, and pulmonary function occurred. Multisystem organ failure precluded further surgery. The patient died 19 days after the initiation of support.
Patient 3
A 59-year-old woman (157 cm, 64 kg) was admitted to another institution for dyspnea. An episode of supraventricular tachycardia was detected, for which she was treated with adenosine and released. One week later, the patient had a syncopal episode accompanied by chest pain and was diagnosed with complete heart block. Inotropic support was initiated and a pacemaker was implanted. Further deterioration of left ventricular function (EF <0.25) and the development of cardiogenic shock led to transfer to our institution for consideration for cardiac transplantation.
Upon the patient's arrival, inotropic support was maximized. Her condition failed to improve, so an IABP was inserted. The patient then underwent implantation of the BVS 5000 mechanical assist device. Right ventricular function was worse than left ventricular function, necessitating biventricular mechanical support. This was followed by removal of the IABP. Myocardial biopsy revealed giant-cell myocarditis (Figs. 3E & F).
Eight days after implantation, a hemothorax was observed in the right pleural space and was evacuated. The left heart recovered partially, but an attempt to wean the patient from the right ventricular assist device revealed that the right ventricle had failed to recover. After 8 days, the patient was listed for transplantation. At 18 days after implantation, a donor heart became available and the patient underwent orthotopic heart transplantation (Figs. 3G & H) and removal of the biventricular assist device. The patient received the donor heart of a 51-year old man who had died of blunt head trauma. The ejection fraction of this heart was 0.68, and a coronary angiogram was not available.
Immunosuppression was carried out with prednisone, mycophenolate mofetil, and oral tacrolimus. The patient recovered uneventfully and all biopsies for the first 6 months were clear for both rejection and GCM. An episode of severe rejection occurred at 7 months after transplantation due to temporary reduction of immunosuppression to treat a transient leukopenia and a urinary tract infection. The patient required stabilization with an IABP and was given antithymocyte globulin and intravenous corticosteroids. Biopsy did not reveal evidence of recurrent GCM. She responded well to treatment and improved symptomatically, with an ejection fraction of 0.45–0.50. Another episode of leukopenia and a neutropenic fever occurred 6 months later, and these were treated successfully with antibiotics. Her latest left ventricular ejection fraction is 0.50–0.55.
Immunosuppression
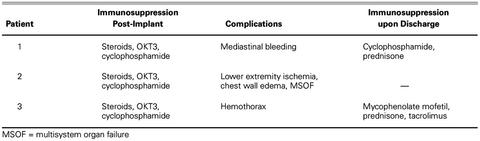
In all 3 patients, congestive heart failure due to GCM was treated primarily with immunosuppression, as described in Table I. This therapy consisted of administration of steroids, OKT3, and cyclophosphamide while on the ventricular assist device. Upon discharge, Patient 1 was treated with cyclophosphamide and prednisone. Patient 3 was given mycophenolate mofetil, prednisone, and oral tacrolimus after transplantation. (See Table II for other details of treatment.)
TABLE I. Immunosuppression Regimen and Complications for All 3 Patients while on the BVS 5000, and Immunosuppression upon Discharge
TABLE II. Details of Treatment during Hospital Course for All 3 Patients

Discussion
Giant-cell myocarditis is a rare and frequently lethal disease that tends to affect otherwise healthy adults. 1 Some of the 1st signs may include dyspnea, fatigue, and syncope, which are also indicative of many other medical conditions. It has been hypothesized that GCM is a form of autoimmune myocarditis that can develop into dilated cardiomyopathy. 7 Hanawa and colleagues induced GCM in a rat model and showed that it is T-cell mediated. 8 We therefore considered that it might respond to T-cell immunosuppression, as opposed to immunosuppressive regimens intended for antibody-mediated conditions. In our study, all 3 patients were treated with immunosuppression while on the assist device, as described in Table I. A recent study suggests that the average transplant-free survival period of GCM patients treated with immunosuppression is more than 4 times longer than that of patients who receive no immunosuppressive therapy (12.6 mo vs 3 mo). 9 In Patient 1, recovery with immunosuppression was enough to obviate the need for heart transplantation.
Transplantation has also been shown as a possible method of treatment, yielding a 5-year survival rate of 71%, despite a 25% rate of giant-cell infiltration into the donor heart. 9,10 It has been estimated that one third of the 25% of recurrences may be lethal. 9,10 This, however, does not consider the many recent advances in immunosuppression.
A recent report that analyzed data in the Multicenter Giant Cell Myocarditis Registry found ventricular assistance to be an effective bridge to transplantation for patients with GCM-induced heart failure. 11 The success rate of bridging GCM patients with a VAD, at 78%, is similar to that of other VAD bridges. Our use of VAD in the treatment of giant cell myocarditis had 3 different results. Patient 1 was initially implanted with mechanical biventricular support as a life-saving treatment for a presumed diagnosis of viral myocarditis. Upon recovery of cardiac function, his only symptom was several episodes of ventricular tachycardia, which were treated successfully with medications and an automatic implantable defibrillator. His unexpected recovery suggests that mechanical support may, in certain cases, be considered as a bridge to recovery for GCM. The present case is, to our knowledge, the first reported in the literature of ventricular support leading to cardiac recovery after diagnosis of GCM. This resembles findings that we previously reported in reviewing the treatment of acute myocarditis with the BVS 5000. 3 As we implied in that earlier report, it is possible that the use of mechanical circulatory assistance enables the metabolic energy of the heart to be used for repair. Mechanical assistance, supplemented by immunosuppression, may retard or reverse the progression of GCM, obviating the need for transplantation. It is also noteworthy to observe that all 3 of our patients required biventricular support, reflecting the nature of GCM, which is likely to cause biventricular disease. This is in contrast with ischemic cardiomyopathy, which often spares the right ventricle.
We thought that Patient 3 could recover without a heart transplant, but we gave up that hope when no progress was observed after initial partial recovery. Our experience with acute myocarditis or acute postcardiotomy failure (although a different disease process) has been that most of the recovery occurs within 7 to 10 days after initiation of mechanical assistance. 12 This particular patient posed several challenges to conversion to an implantable device. As a consequence of acute heart failure, she had a small left ventricular size, which made apical cannulation more difficult. Also, she had worse ventricular failure on the right than on the left and needed biventricular support. Her small body size and chest made implantation of a biventricular device difficult, but those traits were in her favor regarding transplantation, since donor hearts are usually more available for smaller recipients. We avoided a long-term device. A nonstandard* donor heart that was otherwise going to be unused became available, and we chose to use it, knowing that our patient would be immunosuppressed.
In their study, Brilakis and associates reported the 1-year post-transplant survival in patients with GCM who underwent transplantation without any VAD to be higher than in those who were bridged with a VAD. 11 This finding might reflect the poor pretransplant status of the VAD patients and the greater risk that this surgical procedure holds for them. The case of Patient 2 highlights the importance of early treatment and the many potential hazards of rapidly progressive congestive heart failure. This patient was not implanted with a VAD until 3 months after the onset of symptoms. He likely arrived at our institution with compromised circulation to his right leg that went undetected until after he was implanted with the ventricular assist device. His condition was further complicated by the chest-wall edema that he developed during the surgical procedure and the sepsis and multisystem organ failure that followed.
A recent report has found survival in patients with fulminant myocarditis to be significantly greater than in patients with acute nonfulminant myocarditis. 13 It has been suggested that fulminant myocarditis is a distinct clinical entity with a potentially excellent long-term prognosis. 13 Another group reported a GCM patient who had been in cardiogenic shock but completely recovered after conventional immunosuppression. 14 They inferred that GCM is a heterogeneous disease with 1 form that can recover with immunosuppressive therapy, and another form that cannot recover and requires cardiac transplantation. One may hypothesize from these reports that a rapidly progressive acute inflammatory disease, since it is detected earlier, may be less damaging than a slowly progressive one, provided that circulatory support and immunosuppression are begun in time. It is quite possible that chronic inflammatory changes greatly reduce the potential for recovery from a slowly progressive disease.
Patients 1 and 3 had the most fulminant courses before VAD implantation. Patient 1 had been experiencing respiratory symptoms and fatigue for only 5 days before he was diagnosed with congestive heart failure and transferred to our institution. Patient 3 was diagnosed with complete heart block and transferred to our institution 2 weeks after the onset of cardiac symptoms. In contrast, Patient 2 was not transferred to our institution until 3 months after the onset of respiratory symptoms. His initial clinical course (before transfer) suggests that he had a less fulminant form of GCM. For such patients, in whom cardiac recovery is thought to be less likely, urgent transplantation or the use of an implantable VAD may be the best course of initial treatment.
Even though cardiac recovery was not observed after VAD support in Patient 3, her survival suggests that mechanical support could be a viable option as a bridge to transplantation in patients with GCM. As a pulsatile device that avoids left ventricular cannulation, the BVS 5000 is ideally suited for short-term support in patients with GCM. It is inexpensive as an initial treatment and is rapidly deployable, since it is de-aired by the perfusionist. Patient 1 was weaned from mechanical assistance after function recovered. If Patient 2 had not developed sepsis and unrecoverable end organ system failure, the BVS 5000 would have served as an ideal bridge to implantable mechanical support or an artificial heart. In Patient 3, who was quite small, a nonstandard donor heart became available as an endpoint to a very short-term bridge to transplant, avoiding an additional procedure. This patient is currently doing well despite receiving a nonstandard donor heart. This last approach is obviously not possible for all patients but can be successful in selected cases.
Footnotes
*The donor heart was deemed nonstandard following the criteria set by our institution as reported in previous publications. 3 These criteria include the inability to perform an angiogram on a donor heart that is more than 40 years of age.
Address for reprints: Daniel Marelli, MD, Director, Heart Transplant Program, University of Kansas Medical Center, 3901 Rainbow Blvd., G630, Kansas City, KS 66160
E-mail: dmarelli@kumc.edu
Presented in part at the annual meeting of the American Society of Internal Artificial Organs; New York, NY; June 2001.
References
- 1.Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997;336(26):1860–6. [DOI] [PubMed]
- 2.Gries W, Farkas D, Winters GL, Costanzo-Nordin M. Giant cell myocarditis: first report of disease recurrence in the transplanted heart. J Heart Lung Transplant 1992;11(2 Pt 2):370–4. [PubMed]
- 3.Marelli D, Laks H, Amsel B, Jett GK, Couper G, Ardehali A, et al. Temporary mechanical support with the BVS 5000 assist device during treatment of acute myocarditis. J Card Surg 1997;12:55–9. [DOI] [PubMed]
- 4.Marelli D, Laks, H, Fazio D, Hamilton MA, Fonarow GC, Meehan DA, Mariguchi JD. Mechanical assist strategy using the BVS 5000i for patients with heart failure. Ann Thorac Surg 2000;70:59–66. [DOI] [PubMed]
- 5.Champsaur G, Ninet J, Vigneron M, Cochet P, Neidecker J, Boissonnat P. Use of the Abiomed BVS System 5000i as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg 1990;100:122–8. [PubMed]
- 6.Jett GK. ABIOMED BVS 5000. Experience and potential advantages. Ann Thorac Surg 1996;61:301–4,311–3. [DOI] [PubMed]
- 7.Kodama M, Hanawa H, Saeki M, Hosono H, Inomata T, Suzuki K, Shibata A. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ Res 1994;75(2):278–84. [DOI] [PubMed]
- 8.Hanawa H, Inomata T, Okura Y, Hirono S, Ogawa Y, Izumi T, et al. T cells with similar T-cell receptor beta-chain complementarity-determining region 3 motifs infiltrate inflammatory lesions of synthetic peptides inducing rat autoimmune myocarditis. Circ Res 1998;83(2):133–40. [DOI] [PubMed]
- 9.Cooper LT Jr. Giant cell myocarditis: diagnosis and treatment. Herz 2000;25(3):291–8. [DOI] [PubMed]
- 10.Nieminen MS, Salminen US, Taskinen E, Heikkila P, Partanen J. Treatment of serious heart failure by transplantation in giant cell myocarditis diagnosed by endomyocardial biopsy. J Heart Lung Transplant 1994;13:543–5. [PubMed]
- 11.Brilakis ES, Olson LJ, Berry GJ, Daly RC, Loisance D, Zucker M, Cooper LT Jr. Survival outcomes of patients with giant cell myocarditis bridged by ventricular assist devices. ASAIO J 2000;46(5):569–72. [DOI] [PubMed]
- 12.Tsai FC, Marelli D, Laks H, Moriguchi J, Sopher M, Bresson J, et al. Short-term bridge to heart transplant using the BVS 5000 external ventricular assist device. Am J Transplant 2002;2(7):646–51. [DOI] [PubMed]
- 13.McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 2000;342:690–5. [DOI] [PubMed]
- 14.Frustaci A, Chimenti C, Pieroni M, Gentiloni N. Giant cell myocarditis responding to immunosuppressive therapy. Chest 2000;117(3):905–7. [DOI] [PubMed]