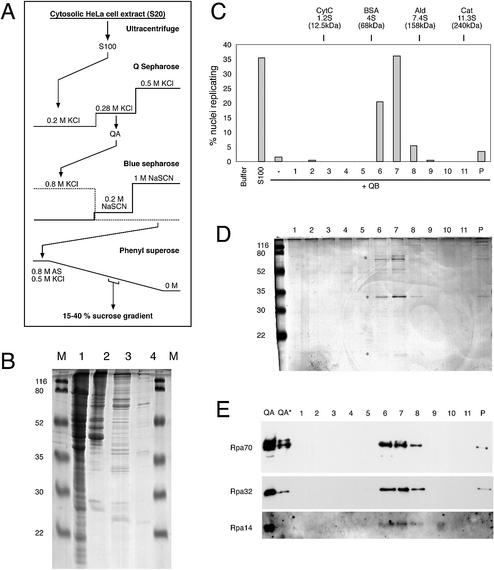
Figure 2.
Purification of the initiation factor present in fraction QA. (A) Schematic diagram of purification procedure. See Materials and Methods for details. (B) Protein analysis of key purification steps. Unfractionated S100 extract (100 µg protein, lane 1), fraction QA (20 µg protein, lane 2), Blue Sepharose eluate (10 µg protein, lane 3) and Phenyl Superose eluate (<1 µg, lane 4) were loaded on a 15% polyacrylamide gel. Proteins were visualised by staining with Coomassie brilliant blue. Molecular masses of marker proteins (M) are indicated. (C–E) Final purification and identification of RPA as the initiation activity present in fraction QA by sucrose gradient centrifugation. (C) Quantitative analysis of initiation of DNA replication. Nuclei from mimosine-arrested EJ30 cells were incubated in 35 µg of fraction QB supplemented with a 20 µl volume of the sucrose gradient fractions (1–11) and the pelleted material (P) as indicated. Incubations in elongation buffer (buffer) or 100 µg of unfractionated extract (S100) were used as controls. Percentages of replicating nuclei of a representative experiment are shown. (D) Protein analysis of fractions of the sucrose gradient step. A 20 µl volume of each sucrose gradient fraction (1–11) and the pelleted material (P) were separated on a 15% polyacrylamide gel and stained with silver salts. Molecular masses of marker proteins (M) are indicated. Asterisks denote the position of Rpa70, 32 and 14 subunits. (E) Identification of RPA by western blot analysis. A 20 µl volume of each sucrose gradient fraction (1–11 and P) and 15 and 5 µg (*) of fraction QA were separated on a 15% polyacrylamide gel and analysed by western blotting. The membrane was sequentially probed with a mixture of monoclonal antibodies 70A, 70B and 70C (Rpa70) (36), monoclonal antibody 34A (Rpa32) (36) and polyclonal antibody pAb-RPA1 (visualising Rpa14; see Fig. 4A for a characterisation of this polyclonal antibody).