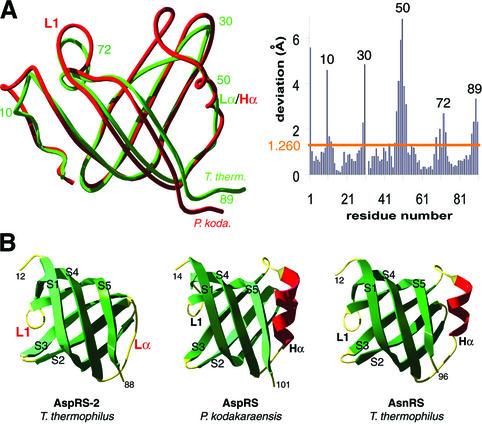
Fig. 6. Comparison of anticodon-binding domains in T.thermophilus AspRS-2, P.kodakaraensis AspRS and T.thermophilus AsnRS. (A) Superimposition of the two domains in Thermus (green) and Pyrococcus (red) AspRSs (left) and r.m.s. deviations (right). (B) Ribbon representation of the anticodon-binding domain in AspRS-2 (left), Pyrococcus AspRS (middle) and Thermus AsnRS (right). Notice in Pyrococcus AspRS and in Thermus AsnRS a standard OB-fold formed by a five-stranded β-barrel with an α-helix (Hα displayed in red) between strands S3 and S4. In AspRS-2, α-helix Hα is replaced by the Lα loop. Loops and strands are coloured in yellow and green, respectively; Lα and L1 loops that are specific to AspRS-2 are emphasized by red labels (notice that L1 corresponds to L45 in the conventional OB-fold nomenclature; Murzin, 1993).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.