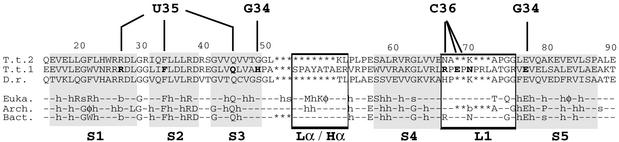
Fig. 7. Peculiarities in the anticodon-binding domain of AspRS-2 from T.thermophilus as revealed by sequence comparison with other AspRSs. (T.t.2, T.t.1) AspRSs from T.thermophilus, (D.r.) D.radiodurans and (Euka., Arch., Bact.) consensus sequences from eukarya, archaea and eubacteria. (–) Non-conserved residues; semi-conserved residues with φ, h, a, b, s representing, respectively, aromatic, hydrophobic, acid, basic and small (Gly, Ala, Ser) side chains; (*) missing amino acids. Residues belonging to β-strands (S1, S2, S3, S4 and S5) of the OB-fold are on a grey background. Lα/Hα and L1 regions are boxed. Amino acids in AspRS-1 that contact the three anticodon identity determinants of tRNAAsp (G34, U35 and C36) are in bold. Notice the differences in length and sequence in L1.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.