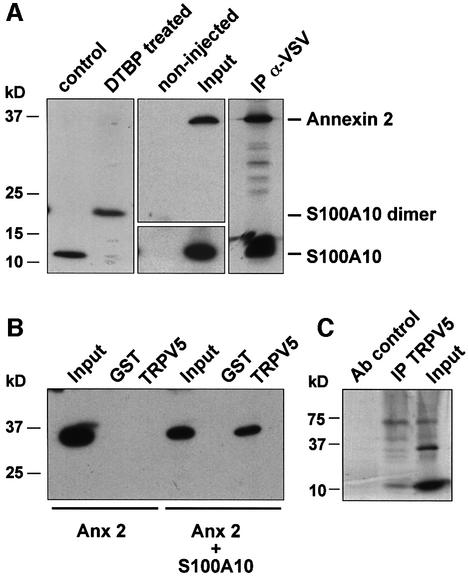
Fig. 2. Annexin 2 interacts with TRPV5 via S100A10. Xenopus laevis oocytes were injected with S100A10 or co-injected with annexin 2 and VSV-tagged S100A10 cRNAs. (A) Lysates of S100A10 cRNA-injected oocytes were treated with DTBP and analyzed by immunoblot. The chemically cross-linked S100A10 band runs at 23 kDa, exactly the expected size of a VSV-tagged S100A10 dimer. Homogenates of non-injected and oocytes co-injected with S100A10 and annexin 2 cRNAs were subjected to immunoprecipitation using monoclonal anti-VSV antibodies. Annexin 2 co-immunoprecipitated with S100A10 as was visualized by autoradiography of the metabolically labeled proteins. As a control, the expression of S100A10 and annexin 2 in the co-injected oocytes was demonstrated by immunoblot analysis to demonstrate that the precipitated proteins were of the correct size. (B) Homogenates of annexin 2 cRNA-injected or S100A10 and annexin 2 cRNA-co-injected oocytes were incubated with GST alone or GST fusion protein containing the TRPV5 C-terminal tail immobilized on glutathione–Sepharose 4B beads. The association of annexin 2 with TRPV5 in the presence of S100A10 was demonstrated by immunoblot using a monoclonal anti-annexin 2 antibody. (C) Full-length annexin 2, S100A10 and TRPV5 were in vitro translated using a reticulocyte lysate system in the presence of canine microsomal membranes. S100A10 and annexin 2 were co-immunoprecipitated with TRPV5 confirming the formation of a TRPV5–S100A10–annexin 2 complex.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.