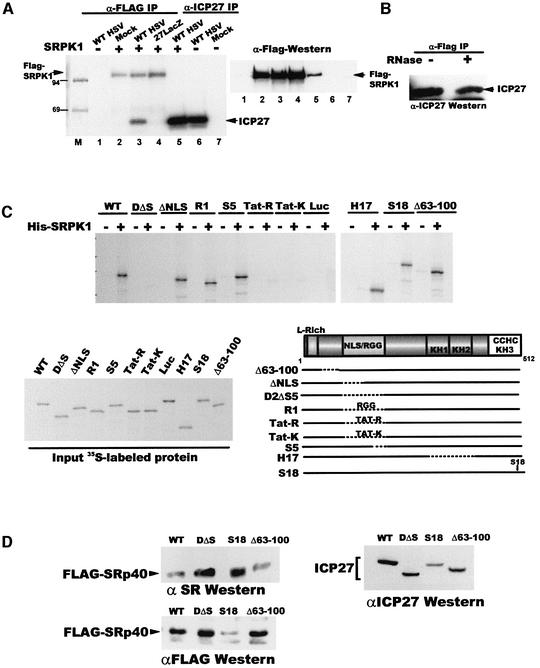
Fig. 5. ICP27 interacts with SRPK1. (A) Cells were transfected with pFlag-SRPK1 (lanes 2–5) or a control plasmid (lanes 1, 6 and 7) and extracts were prepared from transfected cells that were mock-infected (lanes 2 and 7), infected with WT HSV-1 (lanes 1, 3, 5 and 6) or 27-LacZ (lane 4) and labeled with 32Pi from 1 to 5 h after infection. Immunoprecipitation was performed with anti-Flag (lanes 1–4) or anti-ICP27 (lanes 5–7). Radiolabeled proteins (left) and immunoblot analysis with anti-Flag antibody (right) are shown. (B) Extracts from cells transfected with pFlag-SRPK1 or pCMV-ICP27 were treated with RNase (+) or buffer (–) and then mixed. Immunoprecipitation was performed with anti-Flag and the blot was probed with anti-ICP27 antibody. (C) In vitro binding assays were performed with His-SRPK1 and in vitro-translated WT ICP27 or mutant proteins as indicated. Input [35S]methionine-labeled proteins and a schematic showing the positions of the mutations are shown. (D) Cells were co-transfected with Flag-Srp40 and plasmids expressing WT ICP27, D2ΔS5, S18 or Δ63–100. Nuclear extracts were fractionated and western blot analysis was performed with anti-SR antibody (upper left panel), anti-Flag (lower left panel) and anti-ICP27 (right panel).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.