Abstract
The γ-melanocyte-stimulating hormone (γ-MSH) is a natriuretic peptide derived from the N-terminal region of proopiomelanocortin (POMC). Evidence suggests that it may be part of the coordinated response to a low-sodium diet (LSD). We tested the effect of the HSD (8% NaCl) compared with LSD (0.07%) on mean arterial pressure (MAP) in mice with targeted disruption of the PC2 gene (PC2–/–), necessary for processing of POMC into γ-MSH, or the melanocortin receptor 3 gene (Mc3r–/–; the receptor for MSH). In wild-type mice, HSD for 1 week did not alter MAP versus LSD mice, but plasma γ-MSH immunoreactivity was more than double the LSD value. In contrast, in PC2–/– mice, MAP on the LSD was not greater than in wild-type mice, but plasma γ-MSH was reduced to one-seventh the wild-type value. On the HSD, MAP rose to a markedly hypertensive level while plasma γ-MSH concentration remained severely depressed. Intravenous infusion of γ-MSH (0.2 pmol/min) for 30 min to PC2–/– mice after 1 week of HSD lowered MAP from hypertensive levels to normal; infusion of α-MSH at the same rate had no effect. Injection of 60 fmol of γ-MSH into the lateral cerebral ventricle of hypertensive mice also lowered MAP to normal. Administration of a stable analogue of γ-MSH intra-abdominally by microosmotic pump to PC2–/– mice prevented the development of hypertension when ingesting the HSD. In mice with targeted disruption of the Mc3r gene, the HSD also led to marked hypertension accompanied by elevated plasma levels of γ-MSH; infusion of exogenous γ-MSH to these mice had no effect on MAP. These results strongly suggest that PC2-dependent processing of POMC into γ-MSH is necessary for the normal response to the HSD. γ-MSH deficiency results in marked salt-sensitive hypertension that is rapidly improved with exogenous γ-MSH through a central site of action. α-MSH infused at the same rate had no effect on MAP, indicating that the hypertension is a specific consequence of impaired POMC processing into γ-MSH. Absence of Mc3r produces γ-MSH resistance and hypertension on the HSD. These findings demonstrate a novel pathway mediating salt-sensitivity of blood pressure.
Introduction
Numerous neural and humoral systems interact to control total body sodium content, body fluid volumes, and blood pressure through the regulation of urinary sodium excretion (UNaV). These include antinatriuretic pathways such as the renin-angiotensin system, aldosterone and sympathetic nerve activity to the kidneys, and natriuretic pathways such as the natriuretic peptide system (1, 2). Recent evidence has indicated that other natriuretic systems are involved in regulating UNaV, including dopaminergic activity, the kallikrein-kinin system, and other peptide hormonal pathways (2–4). Among these, evidence has been presented that the natriuretic peptide γ-melanocyte-stimulating hormone (γ-MSH) participates in the integrated response to a high intake of dietary sodium: rats ingesting a high-sodium diet (HSD) for 1 week or longer have a marked increase in plasma γ-MSH concentration as well as an increase in the content of γ-MSH in the neurointermediate lobe (NIL) of the pituitary, site of γ-MSH secretion into the circulation (5, 6). Moreover, the HSD also increases the abundance of proopiomelanocortin (POMC) mRNA in the NIL (5, 6); POMC is the ACTH/β-endorphin prohormone, the processing of which in NIL gives rise to γ-MSH (7). Two enzymes involved in POMC processing are the protein proconvertases PC1 and PC2 (7, 8); in the NIL, their action is carefully regulated in a temporal sequence, with PC1 initially cleaving POMC into the large peptides ACTH, β-lipotropin, and a large N-terminal fragment, and PC2 then generating the smaller peptides α-MSH, γ-MSH, β-endorphin, and others (7). The mRNA and protein abundance of these enzymes are also coordinately upregulated by the HSD (6). Since physiologic concentrations of γ-MSH are natriuretic (9, 10), these observations in aggregate suggest that this system may participate in the integrated response to circumstances of dietary sodium excess.
The recent availability of mice with targeted disruption of genes relevant to γ-MSH synthesis and action offers the opportunity to evaluate more definitively the role of this peptide in sodium metabolism (11, 12). γ-MSH exerts its cellular actions by interacting with one of five identified melanocortin receptors, the MC3R (13), and mice with targeted deletion of this gene have been reported (11). In addition, mice with disruption of the PC2 gene have been described (12); its absence would be predicted to result in γ-MSH deficiency because it is necessary for the cleavage of larger POMC-derived peptides into γ-MSH (7, 8). We used these genetically altered mouse strains to examine the importance of γ-MSH in the integrated response to changes in dietary sodium intake. Our results indicate that PC2 deficiency leads to salt-sensitive hypertension that is corrected by infusion of γ-MSH but not by infusion of the closely related POMC-derived peptide α-MSH. MC3R-deficient mice also develop salt-sensitive hypertension, which, in contrast, cannot be corrected by infusion of γ-MSH.
Methods
Mice heterozygous for targeted disruption of the PC2 gene, as described by Furuta et al. (12), were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA), and a breeding colony was established in the transgenic mouse barrier facility at San Francisco General Hospital. These mice were created on a background of C57BL/6J. PC2–/– mice exhibit abnormalities in pancreatic islet hormone processing and have significantly reduced blood sugar concentration and slightly reduced growth rates compared with wild-type mice, but are otherwise phenotypically normal (12). A breeding colony was also established using mice heterozygous for deletion of the MC3R gene as developed by us (11); these mice were also developed on a C57BL/6J background. The knockout mice exhibit a unique metabolic syndrome characterized by an increase in adipose tissue mass without obesity and with reduced energy expenditure (11). We also established a breeding colony using mice heterozygous for the MC4R gene deletion courteously provided by Dennis Huszar, Millenium Pharmaceuticals Inc. (Cambridge, Massachusetts, USA) (14). These knockout mice exhibit an obese phenotype with an increase in adipose tissue, hyperphagia, and insulin resistance (14). For each knockout strain, wild-type littermates were used as controls. Mice were housed in cages and fed a nutritionally complete diet with normal (0.44%) sodium content until entered into the dietary manipulations described below. All protocols were reviewed and approved by the Committee on Animal Research of University of California, San Francisco.
DNA extraction and PCR amplification.
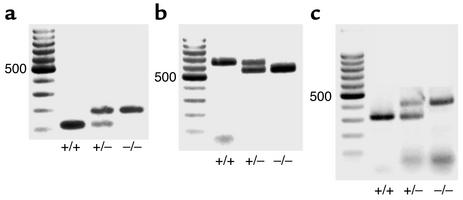
Mice were genotyped at weaning by PCR amplification of DNA extracted from tail tissue using primers described in the original publications (11, 12, 14). DNA was extracted from tail biopsies using the DNeasy Tissue Kit (QIAGEN Inc., Valencia, California, USA), according to the manufacturer’s instructions. Polymerase chain amplification was carried out using HotStarTaq DNA polymerase (QIAGEN Inc.) and the relevant primers for 30 cycles under the cycling conditions reported (11, 12, 14). Amplification products were electrophoresed on 1.4% agarose gels and identified by their size (Figure 1).
Figure 1.
Representative gels showing PCR amplification products for targeted deletions of (a) PC2, (b) Mc3r, and (c) Mc4r genes as described in Methods. +/+, wild type; +/–, heterozygous; –/–, homozygous knockout. Left column is size ladder; 500 indicates 500 bp.
Dietary treatment.
Mice 6 weeks of age and older were placed on either the HSD (8% NaCl; Purina Mills Inc., St. Louis, Missouri, USA) or an otherwise nutritionally identical low-sodium diet (LSD; 0.07%, Purina Mills Inc.) for 7–10 days until they underwent acute study.
Acute experimentation.
On the day of study, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (15 mg/kg) and placed on a heated operating table. A tracheotomy was performed, the carotid artery identified through a small paratracheal incision, and a tapered polyethylene catheter inserted for measurement of arterial pressure via a P23id Statham blood pressure transducer attached to a direct writing recorder (Model 7D; Grass-Telefactor, West Warwick, Rhode Island, USA). A similar catheter was placed in a femoral vein for infusion of solutions. In some mice a flanged polyethylene catheter was sutured into the dome of the urinary bladder via a suprapubic incision for the timed collection of urine. After completion of this preparative surgery, the mice received an infusion of normal saline containing 2 g glucose and 2.5 g BSA per 100 ml, 0.7% body weight over 15 min, to replace surgical fluid losses. This same vehicle solution was infused at 2.5 μl/min for the duration of the experiment. Blood pressure was then measured over the subsequent 30 min, and an average value of the electrical mean taken as mean arterial pressure (MAP) for that animal. In some experiments, the mice were then exsanguinated, the blood processed as described below, and the animal sacrificed.
In other experiments, the normal saline vehicle was infused intravenously at 2.5 μl/min while urine was collected for 30 min. The intravenous infusion was then changed to vehicle containing α-MSH to deliver 0.2 pmol/min for a second 30-min period. The infusion was changed again to vehicle containing γ-MSH at 0.2 pmol/min for a third 30-min period, following which a large blood sample was obtained and the mouse sacrificed. Urine was collected during each 30-min interval for determination of UNaV, and MAP was determined at the end of each period. In three studies only γ-MSH was infused.
In an effort to determine the effect of anesthesia on MAP, we studied five MC3R knockout mice fed the HSD for 1 week. They were anesthetized with ketamine/xylazine as described and a catheter placed in the carotid artery. The catheter was then tunneled subcutaneously to exit between the scapulae and secured with a suture. MAP was recorded while the mice were still under anesthesia and then placed in cages until the anesthesia wore off and they were moving freely. MAP was measured again at this time, 6 hours after injection of the anesthesia.
In additional experiments, we tested the effect of γ-MSH injected directly into the cerebroventricular system of PC2+/+ and PC2–/– mice fed the HSD for 1 week. Mice were anesthetized as described, a tracheotomy tube placed, and the carotid artery and jugular vein catheterized. The animal was then placed on its ventral surface. The scalp was incised in the midline and a 26-gauge needle drilled through the skull to a depth of 2.5 mm, as described by Ackermann and Azizi (15), 0.5 mm caudal and 1 mm lateral to the bregma. Placement of the tip of the needle in the lateral ventricle was confirmed at the end of the experiment by injection of 3 μl India ink through the needle followed by brain dissection; results from only those mice in which the ink was confined to the ventricular space were accepted for analysis. The experiment was begun 20 min after the completion of surgery. Baseline MAP was recorded for 10 min, at which time γ-MSH, 60 fmol in 5 μl of normal saline followed by a flush of 10 μl, was injected intravenously. MAP was observed for another 10 min, when 20 fmol γ-MSH in a volume of 5 μl was injected intracerebroventricularly. MAP was again recorded for 10 min, and a second dose of γ-MSH, 60 fmol in 5 μl, was injected. MAP was recorded for a final 10 min, after which the India ink was injected, the mouse sacrificed, and the brain dissected.
Chronic studies.
The effect of continuous γ-MSH administration on MAP during ingestion of the HSD was tested in ten PC2–/– mice. Under Brevital anesthesia (50 mg/kg intraperitoneally), Alzet microosmotic pumps (Model 1007D; Durect Corp., Cupertino, California, USA) were placed intra-abdominally through a midline surgical incision. In half the mice, the pumps were loaded with vehicle while in the other half they were loaded with vehicle containing the stable γ-MSH analogue [Nle3,D-Phe6]γ-MSH (NDP-γ-MSH) (16, 17) to deliver 12 pmol/h in a volume of 0.5 l/h directly into the peritoneal space. The wounds were closed with sutures and the mice returned to their cages after recovery from anesthesia. They were placed on the HSD, and 1 week later underwent acute experimentation as described above for measurement of MAP.
Radioimmunoassays.
Blood was collected in chilled Vacutainer tubes (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) containing EDTA and 500 KIU aprotinin and centrifuged immediately at 4°C. The plasma was decanted and stored at –70°C until assayed for γ-MSH concentration, plasma renin activity (PRA), and plasma aldosterone concentration. For the γ-MSH assay, samples were thawed on ice and extracted through Sep-Column chromatography cartridges (Penninsula Laboratories Inc., San Carlos, California, USA); samples from three mice were pooled to obtain sufficient plasma for assay. The cartridges were prepared by wetting with 3 ml of solvent A — acetonitrile/water/trifluoroacetic acid (TFA; 80:19.9:0.1) — and with 3 ml of 0.1% TFA. The cartridge was then washed with 10 ml of 0.1% TFA and eluted with solvent A. Eluates were lyophilized and stored at –70°C until assay, as described previously (5, 6, 16), using a commercially available RIA kit (Peninsula Laboratories Inc., San Carlos, California, USA) with 125I-γ2-MSH as tracer. Results are expressed as femtomoles per milliliter of plasma. The characteristics of this assay have been described (16). PRA was determined in unextracted plasma pooled from two mice by RIA of angiotensin I (A1) generated after incubation of plasma at 37°C using a commercial kit (DiaSorin, Stillwater, Minnesota, USA) according to the manufacturer’s instructions. Plasma aldosterone concentration was measured in 200 μl of unextracted plasma using the DPC Coat-A-Count kit (Diagnostic Products Corp., Los Angeles, California, USA), according to the instructions included in the kit.
Statistical analysis.
Data are presented as means plus or minus SEM. Analysis was performed using GraphPad InStat software (GraphPad Software Inc., San Diego, California, USA). Comparisons among groups were carried out using one-way or repeated measures ANOVA, as appropriate, with the Bonferroni post-hoc test when significant differences were recognized. Groups that did not have equal standard deviations underwent log transformation of the data before carrying out ANOVA. Comparisons between two groups used the unpaired t test. A P value of less than 0.05 was used to indicate the presence of a significant difference.
Results
Genotyping.
Mice underwent genotyping by PCR amplification of DNA extracted from tail biopsies as described in Methods; the results are shown in Figure 1. PC2+/+ mice exhibited the predicted 117-bp product, whereas PC2–/– mice showed a product of 180 bp and heterozygotes displayed both bands, as reported previously (12) (Figure 1a). Mc3r+/+ mice demonstrated an amplification product of 646 bp, while Mc3r null mice showed a band of 550 bp (11) (Figure 1b). Mc4r+/+ mice showed a product of 313 bp, while Mc4r–/– mice displayed a product of 405 bp (14) (Figure 1c).
Effect of dietary sodium intake in PC2–/– mice.
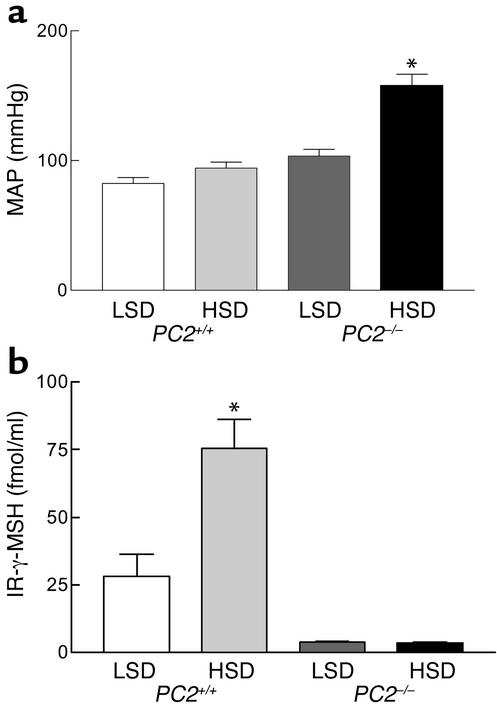
Results of measurements of MAP and plasma γ-MSH concentration in PC2–/– mice and PC2+/+ littermates on the LSD and HSD are shown in Figure 2. On the LSD, MAP averaged 83 ± 5 mmHg (n = 8) in wild-type mice and was statistically unchanged in eight other animals ingesting the HSD (94 ± 5 mmHg; P = NS) (Figure 2a). In PC2–/– mice, MAP on the LSD was somewhat elevated compared with wild-type mice on either the LSD or HSD at 104 ± 5 mmHg, but this value was not significantly greater (one-way ANOVA, P < 0.10). On the HSD, however, PC2 null mice exhibited a marked increase in MAP to 158 ± 9 mmHg (P < 0.001 versus all other groups). Thus, PC2 knockout mice exhibit marked salt-sensitive hypertension. These differences in MAP were related to differences in plasma γ-MSH immunoreactivity. In PC2+/+ mice, plasma concentration of the peptide was 28.1 ± 8.1 fmol/ml during ingestion of the LSD and was markedly increased at 75.6 ± 10.4 fmol/ml after a week of the HSD (P < 0.001) (Figure 2b). In contrast, PC2–/– mice on the LSD had a reduced plasma γ-MSH concentration at 3.9 ± 0.1 fmol/ml (P < 0.10 versus PC2+/+ mice) and did not increase it during ingestion of the HSD (3.5 ± 0.3 fmol/ml; P < 0.001). The HSD leads to a robust increase in plasma γ-MSH concentration in wild-type mice, just as observed in rats (5, 6); absence of PC2 marginally reduces circulating γ-MSH during ingestion of the LSD and completely prevents the increase expected during ingestion of the HSD. Since these last animals were also hypertensive, these observations raise the possibility that the γ-MSH deficiency could in some way be linked to the development of the salt-sensitive hypertension.
Figure 2.
MAP (a) and plasma IR-γ-MSH concentration (b) after 1 week of a LSD or HSD in PC2+/+ and PC2–/– mice. Results are means ± SEM of measurements of MAP in eight mice in each group, as described in Methods, and of IR-γ-MSH in five samples of plasma, each pooled from three mice. *P < 0.001 versus all other values.
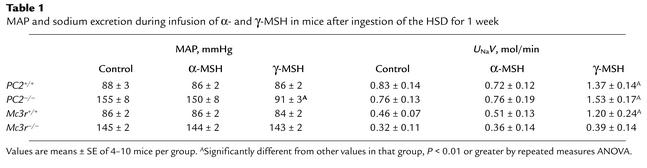
To determine if γ-MSH replacement improves MAP in PC2–/– mice fed the HSD for one week, we infused the peptide intravenously as described in Methods. In five mice, a 30-min control period was followed by a 30-min infusion of α-MSH at 0.2 pmol/min, in turn followed by a 30-min infusion of γ-MSH; in three additional mice, only γ-MSH was infused. The results are shown in Table 1. Infusion of α-MSH had no effect on MAP or UNaV compared with the control period. In marked contrast, subsequent infusion of γ-MSH lowered MAP to the normal range and also approximately doubled UNaV (Table 1). In the three mice infused only with γ-MSH, MAP fell from 146 ± 6 to 103 ± 2 mmHg. Plasma γ-MSH concentration at the end of the infusion was 165 ± 28.4 fmol/ml, a value roughly twice that seen in wild-type mice during ingestion of the HSD. In two mice undergoing vehicle infusion only throughout, MAP was unchanged (143–145 and 158–160 mmHg).
Table 1.
MAP and sodium excretion during infusion of α- and γ-MSH in mice after ingestion of the HSD for 1 week
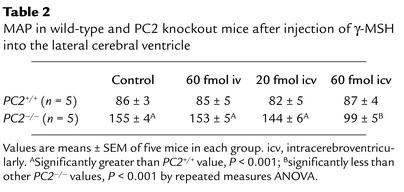
We sought to determine if this hypotensive effect of γ-MSH in hypertensive PC2–/– mice on the HSD occurred through a central or a peripheral site of action. To do this, we injected small doses of the peptide intravenously and into the CNS. The results are presented in Table 2. In PC2+/+ mice (n = 5) who were normotensive after 1 week of the HSD, no significant effect of the peptide on MAP was observed, whether injected intravenously or intracerebroventricularly. PC2–/– were hypertensive; 60 fmol of γ-MSH injected intravenously had no effect on MAP. Injection of 20 fmol of the peptide intracerebroventricularly had an insignificant effect in lowering MAP (153 ± 5 to 144 ± 6 mmHg; P = NS). Injection of 60 fmol, however, caused MAP to fall profoundly to 99 ± 5 mmHg, with P < 0.001 versus all other values. Thus, a dose of γ-MSH that had no effect when administered intravenously restored MAP to near-normal levels when given into the cerebral ventricle.
Table 2.
MAP in wild-type and PC2 knockout mice after injection of γ-MSH into the lateral cerebral ventricle
We also tested the effect of continuous γ-MSH replacement on the development of salt-sensitive hypertension in PC2–/– mice. To do this, we inserted microosmotic pumps intra-abdominally as described in Methods in ten knockout mice. In five mice, the pumps were loaded with vehicle and the other five with NDP-γ-MSH, a stable analogue of γ-MSH with equal natriuretic potency (16). Mice then ingested the HSD for 1 week before undergoing measurement of MAP. In vehicle-administered mice, MAP was 159 ± 5 mmHg, whereas in mice infused with NDP-γ-MSH, MAP was 92 ± 4 mmHg (P < 0.001). Thus, continuous infusion of the γ-MSH analogue prevented the development of hypertension when the PC2 knockout mice were exposed to the HSD.
In five samples of plasma pooled from ten PC2+/+ mice on the LSD for 1 week, PRA was 5.8 ± 1.1 ng A1/ml/h and was suppressed to 2.1 ± 1.3 ng A1/ml/h in five other samples from mice on the HSD (P = 0.0683). Respective values in PC2–/– mice were 4.7 ± 1.3 (LSD) and 2.4 ± 0.9 (HSD) ng A1/ml/h (P = 0.1784). Corresponding values for plasma aldosterone concentration were 170 ± 38.4 (LSD, n = 5) and 65.3 ± 13.7 (HSD, n = 5) pg/ml in PC2+/+ mice (P < 0.05) and 198 ± 50.4 (LSD, n = 4) and 76.8 ± 11.0 (HSD, n = 5) pg/ml in PC2–/– mice (P < 0.05).
Mc3r–/– mice.
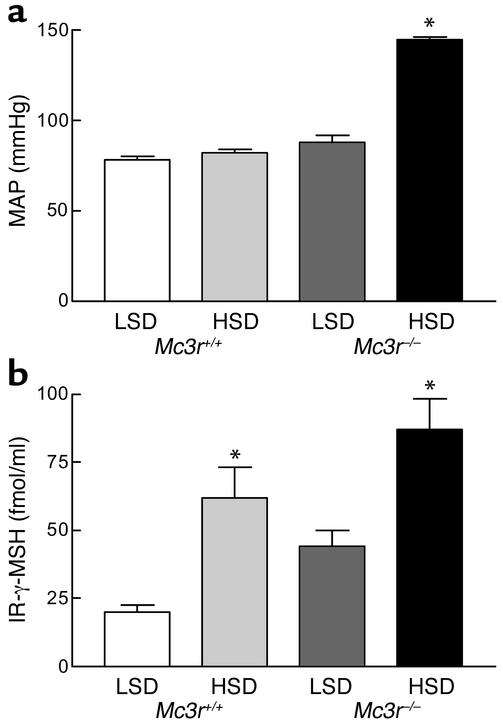
We next examined the effect of 1 week of the HSD on MAP in mice lacking the γ-MSH receptor MC3R. As shown in Figure 3a, Mc3r+/+ mice had a MAP of 78 ± 2 mmHg on the LSD and 82 ± 2 mmHg on the HSD (P = NS). Mc3r–/– mice were normotensive on the LSD (88 ± 4 mmHg; P > 0.05 versus Mc3r+/+), but, like the γ-MSH–deficient PC2–/– mice, were markedly hypertensive on the HSD (145 ± 2 mmHg; P < 0.001 versus all other values). The plasma γ-MSH concentrations in these groups of mice are shown in Figure 3b. Mc3r+/+ mice had values slightly but not significantly lower than those observed in PC2+/+ mice, but, as in PC2+/+ mice, levels on the HSD were triple those on the LSD (61.8 ± 11.6 versus 20.1 ± 2.5 fmol/ml, n = 5 for each group; P < 0.001). Plasma γ-MSH was higher on the LSD than the value observed in wild-type mice (44.4 ± 5.5 fmol/ml, P < 0.01), but still increased on the HSD to 87.2 ± 11.3 fmol/ml (P < 0.05), a level that, however, was not significantly greater than the one seen in Mc3r+/+ mice on the HSD (P < 0.10). Thus, these MC3R knockout mice also developed salt-sensitive hypertension in a manner identical to the PC2 knockout mice, but had higher plasma γ-MSH concentrations rather than the low levels observed in the PC2 knockout mice. This observation is consistent with a hormone-resistance state.
Figure 3.
MAP (a) and plasma IR-γ-MSH concentration (b) after 1 week of a LSD or HSD in Mc3r+/+ and Mc3r–/– mice. Results are means ± SEM of MAP in five mice in each group and of IR-γ-MSH concentration in five samples of plasma, each pooled from three mice. *P < 0.001 versus all other values.
In an effort to determine the effect of the anesthesia on our measurement of MAP, we studied five Mc3r–/– mice fed the HSD for 1 week. MAP was recorded under anesthesia immediately after placement of the arterial catheter and was 149 ± 5 mmHg. The mice were allowed to recover from anesthesia; 6 h later, MAP was again recorded and found to be 142 ± 4 mmHg (P = NS versus value under anesthesia). This indicates that our technique for measuring MAP is not significantly distorted by the anesthetic agents we used.
We also tested the effect of MSH infusion in the Mc3r–/– mice and the results are shown in Table 1. Infusion of neither α-MSH nor γ-MSH into knockout mice on the HSD for 1 week had any effect on MAP or on UNaV, in contrast to results in PC2 knockout mice. These results indicate that the MC3R is necessary for γ-MSH to lower MAP in rodents ingesting the HSD and also is required for the natriuretic action of the peptide.
Mc4r–/– mice.
MAP in five wild-type mice on the LSD was 84 ± 5 mmHg. In five Mc4r–/– mice ingesting the HSD for 1 week, MAP had no statistical difference at 88 ± 7 mmHg. Although Mc4r–/– mice have an obese phenotype with insulin resistance (14), characteristics often associated with an elevated blood pressure, they do not develop salt-sensitive hypertension.
Discussion
The phenomenon of salt sensitivity of blood pressure has been frequently observed, but remains poorly understood, in part because only about one-half of hypertensive individuals exhibit it (18). It is variably thought to result from impaired renal sodium excretion from intrinsic renal disorders, or from dysregulation of the renin-angiotensin system, or heightened activity of the sympathetic nervous system, among other mechanisms (19–21). Despite redundancy in the pathways regulating renal sodium reabsorption and excretion, Mendelian forms of human hypertension caused by single gene defects all impact on renal-sodium handling (22), and it is also noteworthy that single gene deletion studies in rodents have also revealed the phenotype of salt-sensitive hypertension. For example, mice lacking the atrial natriuretic peptide (ANP) gene (23), the bradykinin B2 receptor (24), or the endothelin-B receptor (25) all exhibit salt-sensitive hypertension. The results of our studies indicate that disruption of the γ-MSH system, currently not recognized to interact with those just mentioned, also causes major salt-sensitive hypertension, thereby offering further insight into the pathways by which salt sensitivity may develop as well as arguing for an important role of this system in normal sodium metabolism.
Earlier evidence indicated that the γ-MSH system is responsive to changes in dietary sodium intake. The plasma concentration of the peptide is increased when rats are placed on the HSD (5, 6), and the pituitary content of γ-MSH immunoreactivity as well as the mRNA abundance of POMC also increase after exposure to the HSD (5, 6). These changes are confined to the NIL, the lobe responsible for the processing of POMC into its smaller peptide products, and are accompanied by increases in the mRNA abundance of the processing enzymes necessary for cleavage of POMC into γ-MSH (6). The mouse displays a response comparable to the rat in that plasma γ-MSH concentration more than doubled in wild-type mice on the HSD for 1 week compared with the LSD. These observations all suggest a potential role for γ-MSH as a sodium-regulating hormone; however, they do not demonstrate that functioning of this system is required to maintain normal sodium homeostasis. This issue consequently provided the rationale for studying the effects of γ-MSH deficiency or resistance on sodium metabolism using genetically altered mice.
PC2 null mice exhibited marked salt-sensitive hypertension: MAP during ingestion of the HSD was more than 50 mmHg greater than that observed in wild-type mice or in knockout mice on the LSD (Figure 2). This hypertension was accompanied by a dramatic reduction in basal plasma γ-MSH concentration on the LSD to values only 10% of those seen in wild-type animals and a complete absence of any stimulation of peptide concentration in plasma when challenged with the HSD (Figure 2). Thus, although the residual immunoreactivity in plasma in knockout mice on the LSD may reflect alternate pathways of POMC processing, these alternate pathways do not respond in any measurable way to stimulation with the HSD. These data indicate that PC2 is necessary for most of the basal (unstimulated) level of plasma γ-MSH as well as all of the increase occurring in response to the HSD.
PC2-deficient mice might also be expected to have multiple abnormalities in prohormone processing in view of the widespread distribution of this enzyme and its identified role in peptide hormone secretion (26). Defects in prodynorphin, proglucagon, and prosomatostatin processing have been shown (13, 26–28), and altered POMC processing in the pituitary has been documented (29). It is thus possible that altered secretion of some other peptide involved in the regulation of blood pressure and UNaV could exist besides γ-MSH to account for the development of hypertension while ingesting the HSD. Although this possibility cannot be refuted with certainty until more comprehensive measurements are made of the impact of dietary sodium intake on peptide hormone metabolism, several lines of evidence argue against it. First, infusion of γ-MSH to hypertensive γ-MSH–deficient PC2–/– mice rapidly restored MAP to control levels, implying that the deficiency of the peptide in these mice was in some way responsible for the hypertension. Second, qualitatively and quantitatively similar hypertension developed in Mc3r–/– mice when exposed to the HSD. These mice actually had elevated levels of γ-MSH on both the LSD and the HSD, yet still developed salt-sensitive hypertension. Of the five melanocortin receptors identified to the present, γ-MSH binds with highest affinity to MC3R, which is thought to mediate its actions (13, 30, 31). The occurrence of hypertension in MC3R knockout mice strengthens the contention that hypertension on the HSD results from disruption of the γ-MSH system, whether from impaired production and secretion, as in PC2–/– mice, or from impaired action, as in the receptor-deficient Mc3r–/– mice. Third, if another peptide pathway were involved, it would likely be impaired synthesis and secretion of a vasodilatory and natriuretic peptide such as ANP to cause the hypertension when ingesting the HSD. Although deletion of the ANP gene does result in salt-sensitive hypertension (23), there is no evidence that PC2 is involved in the processing of proANP and secretion of ANP by atrial myocytes (32). The processing of other vasodilatory peptides besides ANP could be involved in our results. Our observation that α-MSH infusion has no effect to lower MAP in hypertensive PC2–/– mice, however, also suggests that the hypertension is a specific consequence of impaired processing of POMC into γ-MSH, and its secretion and action through MC3R, even if the processing of POMC into other smaller peptides is also disrupted in these mice.
The results of these studies also provide new information regarding the natriuretic action of γ-MSH. Although each of the MSH peptides has been known to be natriuretic (9, 10, 33–35), the mechanism of this natriuresis has not been established, either with respect to nephron site of action or the transport system involved. Available data indicate that the natriuresis occurs on intrarenal infusion of the peptide, without change in MAP or glomerular filtration rate, and is interrupted by acute renal denervation (9, 10). The present data indicate that intravenous γ-MSH is natriuretic in PC2–/– mice with intact melanocortin receptors, but not in Mc3r–/– mice lacking the receptor with which the peptide interacts. The failure of α-MSH infusion to cause natriuresis is somewhat surprising given its affinity to MC3R in vitro (13); this lack of natriuretic activity strengthens the contention that γ-MSH is the POMC-derived peptide involved in sodium homeostasis. MC3Rs have been identified in human (36) and rat (37) kidney, and our results demonstrate that it is this receptor that mediates the natriuresis caused by the peptide. Since neither α- nor γ-MSH was natriuretic in Mc3r–/– mice, it does not appear that other renal melanocortin receptors are involved in the natriuresis. Finally, γ-MSH led to equivalent natriuresis in wild-type and PC2–/– mice (Table 1); to the extent that the natriuretic response can be used as an index of receptor activity or expression, this argues against the possibility that deficiency of the hormone led to upregulation of Mc3r in the kidney. This question will obviously have to be addressed in studies directly measuring the renal expression of the melanocortin receptors in these different mouse models.
The mechanism by which the HSD results in hypertension in PC2–/– and Mc3r–/– mice is not clear from these studies. One possibility is that the natriuresis resulting from γ-MSH infusion leads to a reduction in plasma volume and a consequent lowering of MAP. Against this is the rapidity of the fall in MAP with only a doubling of UNaV so that a large reduction in plasma volume would not have occurred. Given our evidence of a central site of action of γ-MSH to lower MAP (Table 2), it may be that this mechanism interacts with the natriuresis in the long-term regulation of MAP. Altered regulation of the renin-angiotensin system should also be considered as a component of the salt-sensitive hypertension we have observed. The limited data we have obtained suggest that PRA and plasma aldosterone concentration are suppressed by the HSD to an equal extent in wild-type and PC2–/– mice, even though, in the case of the former, this suppression was not significant statistically because of the large variance in the samples assayed. Furthermore, there is no evidence of PC2 involvement in prorenin processing (38). Studies examining the effect of an angiotensin receptor antagonist on blood pressure in the hypertensive mice would address more directly any contribution of circulating angiotensin II to the hypertension.
A third mechanism could be activation of the sympathetic nervous system by the HSD in peptide- or receptor-deficient mice. In this formulation, γ-MSH acting through central Mc3r exerts a tonic inhibition of sympathetic outflow developing in response to the HSD; deficiency of the hormone (PC2–/– mice) or blunted responsiveness (Mc3r–/– mice) then allows this increased outflow to go unchecked, resulting in hypertension. Infused γ-MSH could gain access to the CNS via the circumventricular organs that lie outside the blood-brain barrier; the peptide could thereby restore central inhibition of sympathetic outflow. Our observation that intracerebroventricular administration of γ-MSH lowered MAP at a dose that had no effect when given intravenously (Table 2) supports such a mechanism. This hypotensive action of the peptide actually is opposite that of published action of γ-MSH in the CNS, where it has been reported to stimulate sympathetic outflow, increase MAP, and produce tachycardia in most (reviewed in ref. 30, 31, 39) but not all (40) reports. One study, however, has observed a hypotensive and bradycardic action of the peptide when injected directly into the nucleus of the tractus solitarius of the rat (41). Since this is one of the circumventricular organs, it is possible that it mediates the hypotensive effect of the intravenous γ-MSH infusion in hypertensive PC2–/– mice. These conflicting reports, and other data on the central actions of γ-MSH, have led to the speculation that another, as yet unidentified, melanocortin receptor mediates the cardiovascular actions of the peptide (42). This inference notwithstanding, our results clearly show that its hypotensive action in hypertensive, salt-loaded PC2–/– mice occurs through Mc3r, since it is not observed in the Mc3r knockout mice. Although the rapidity with which γ-MSH corrects the hypertension in salt-loaded PC2–/– mice suggests the possibility of a direct effect on resistance blood vessels, the hypotension observed following intracerebroventricular injection of the peptide (Table 2) makes this unlikely as a major mechanism.
The relevance of these observations to human salt-sensitive hypertension remains to be established. In contrast to rodents, humans do not have a clearly defined pituitary intermediate lobe. Cells can be identified in the human pituitary, however, with histochemical and immunocytochemical characteristics of melanotrophs (39, 43–45), and γ-MSH-like immunoreactivity has been identified in human plasma, although not fully characterized (46, 47). Some years ago, Griffing and colleagues proposed that this peptide may be involved in the pathogenesis of some forms of idiopathic hyperaldosteronism (46). Mineralocorticoid-induced hypertension, however, does not seem to account for the salt sensitivity of PC2–/– mice, since plasma aldosterone concentration was suppressed by the HSD in the knockouts to the same extent as in the wild-type mice. The plasma concentration of γ-MSH was also observed to be elevated in patients with severe heart failure (47). Additionally, components of this peptide system are found in many human tissues (30). It thus seems plausible to entertain the possibility that the γ-MSH system may be part of the integrated response to increases in dietary sodium intake and that derangements in this system may be implicated in some cases of salt-sensitive hypertension.
Acknowledgments
We would like to acknowledge the assistance of Baoji Xu, Department of Physiology, University of California, San Francisco, in the conduct of the experiments involving the injection of γ-MSH into the cerebral ventricle. This work was supported by grants DK-58812 and HL-68871 to M.H. Humphreys, DK-56695 to D. Pearce, and DK-55819 and DK-51730 to R.D. Cone, all from the NIH.
Footnotes
See the related Commentary beginning on page 1115.
Andrew A. Butler’s present address is: Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, Louisiana, USA.
Portions of this work were presented at the Annual Meeting of the American Society of Nephrology in Philadelphia, Pennsylvania, USA, November 1–4, 2002. Portions of this work have appeared in abstract form (2002. J. Am. Soc. Nephrol. 13:53A).
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: urinary sodium excretion (UNaV); γ-melanocyte-stimulating hormone (γ-MSH); high-sodium diet (HSD); neurointermediate lobe (NIL); proopiomelanocortin (POMC); prohormone convertase 1 (PC1); melanocortin receptor 3 (MC3R); low-sodium diet (LSD); mean arterial pressure (MAP); [Nle3,D-Phe6]γ-MSH (NDP-γ-MSH); plasma renin activity (PRA); trifluoroacetic acid (TFA); A1 (angiotensin 1); atrial natriuretic peptide (ANP).
References
- 1.Kurtz, I. 2001. Control of sodium excretion. In Textbook of Nephrology. 4th edition. S.G. Massry and R.J. Glassock, editors. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 254–261.
- 2.Humphreys, M.H., and Valentin, J.-P. 2000. Natriuretic humoral agents. In The kidney: physiology and pathophysiology. 3rd edition. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 1371–1410.
- 3.Carey RM. Renal dopamine system. Paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 4.Katori M, Majima M, Hyashi I. Crucial suppressive role of renal kallikrein-kinin system in development of salt-sensitive hypertension. Biol. Res. 1998;31:143–149. [PubMed] [Google Scholar]
- 5.Mayan H, et al. Dietary sodium intake modulates pituitary proopiomelanocortin mRNA abundance. Hypertension. 1996;28:244–249. doi: 10.1161/01.hyp.28.2.244. [DOI] [PubMed] [Google Scholar]
- 6.Chandramohan G, Ni X-P, Kalinyak JE, Humphreys MH. Dietary sodium modulates mRNA abundance of enzymes involved in pituitary processing of proopiomelanocortin. Pituitary. 2001;4:231–237. doi: 10.1023/a:1020746414046. [DOI] [PubMed] [Google Scholar]
- 7.Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J. Biol. Chem. 1993;268:1763–1769. [PubMed] [Google Scholar]
- 8.Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SY, Chaves C, Wiedemann E, Humphreys MH. A γ-melanocyte stimulating hormone-like peptide causes reflex natriuresis after acute unilateral nephrectomy. Hypertension. 1987;10:619–627. doi: 10.1161/01.hyp.10.6.619. [DOI] [PubMed] [Google Scholar]
- 10.Chen X-W, et al. Mechanism of the natriuretic action of γ-melanocyte-stimulating hormone. Am. J. Physiol. 1997;272:R1946–R1953. doi: 10.1152/ajpregu.1997.272.6.R1946. [DOI] [PubMed] [Google Scholar]
- 11.Butler AA, et al. A unique metabolic syndrome causes obesity in the melanocrotin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 12.Furuta M, et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roselli-Rehfuss L, et al. Identification of a receptor for γ-melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann U, Azizi N. Increased central AT1-receptor activation, not systemic vasopressin, sustains hypertension in ANP knockout mice. Am. J. Physiol. 2000;278:R1441–R1445. doi: 10.1152/ajpregu.2000.278.6.R1441. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer TK, et al. 4-Norleucine, 7-D-phenylalanine-α-melanocyte stimulating hormone: a highly potent α-melanotropin with ultralong biological activity. Proc. Natl. Acad. Sci. U. S. A. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni X-I, et al. Prevention of reflex natriuresis after acute unilateral nephrectomy by melanocortin receptor antagonists. Am. J. Physiol. 1998;274:R931–R938. doi: 10.1152/ajpregu.1998.274.4.R931. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N. Engl. J. Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 20.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 21.Muntzel M, Drueke T. A comprehensive review of the salt and blood pressure relationship. Am. J. Hypertens. 1992;5:1S–42S. doi: 10.1093/ajh/5.4s.1s. [DOI] [PubMed] [Google Scholar]
- 22.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 23.John SW, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 24.Alfie ME, Yang X-P, Hess F, Carretero OA. Salt-sensitive hypertension in bradykinin B2 receptor knockout mice. Biochem. Biophys. Res. Commun. 1996;224:625–630. doi: 10.1006/bbrc.1996.1076. [DOI] [PubMed] [Google Scholar]
- 25.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J. Clin. Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274:20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- 27.Berman Y, et al. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J. Neurochem. 2000;75:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- 28.Furuta M, et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J. Biol. Chem. 2001;276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 29.Allen RG, et al. Altered processing of pro-orphanin FQ/nociceptin and proopiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. J. Neurosci. 2001;21:5864–5870. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wikberg JES, et al. New aspects on the melanocortins and their receptors. Pharmacol. Res. 2000;42:393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- 31.Schioth HB. The physiological role of melanocortin receptors. Vitam. Horm. 2001;63:195–232. doi: 10.1016/s0083-6729(01)63007-3. [DOI] [PubMed] [Google Scholar]
- 32.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orias R, McCann SM. Natriuresis induced by alpha and beta melanoctye stimulating hormone (MSH) in rats. Endocrinology. 1972;90:700–706. doi: 10.1210/endo-90-3-700. [DOI] [PubMed] [Google Scholar]
- 34.Hradec J, Horky K. Natriuretic and kaliuretic effect of melanocyte stimulating hormones in hamster. Endocrinol. Exp. 1979;13:145–152. [PubMed] [Google Scholar]
- 35.Lymangrover JR, Buckalew VM, Harris J, Klein MC, Gruber KA. Gamma-2 MSH is natriuretic in the rat. Endocrinology. 1984;116:1227–1229. doi: 10.1210/endo-116-3-1227. [DOI] [PubMed] [Google Scholar]
- 36.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem. Mol. Biol. Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 37.Ni X-P, Bhargava A, Pearce D, Humphreys MH. Dietary sodium intake modulates renal expression of melanocortin 3 receptor (MC3-R) mRNA and protein abundance in the rat. J. Am. Soc. Nephrol. 2002;13:81A. doi: 10.1152/ajpregu.00279.2005. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 38.Laframboise M, et al. Prorenin activation and prohormone convertases in the mouse As4.1 cell line. Kidney Int. 1997;51:104–109. doi: 10.1038/ki.1997.13. [DOI] [PubMed] [Google Scholar]
- 39.Gruber KA, Callahan MF. ACTH-(4–10) through γ-MSH: evidence for a new class of central autonomic nervous system-regulating peptides. Am. J. Physiol. 1989;257:R681–R694. doi: 10.1152/ajpregu.1989.257.4.R681. [DOI] [PubMed] [Google Scholar]
- 40.De Wildt DJ, Krugers H, Kasbergen CM, De Lang H, Versteeg DHG. The hemodynamic effects of γ2-melanocyte stimulating hormone and related melanotropins depend on the arousal potential of the rat. Euro. J. Pharmacol. 1993;233:157–164. doi: 10.1016/0014-2999(93)90362-l. [DOI] [PubMed] [Google Scholar]
- 41.De Wildt DJ, Van Der Ven JC, Van Bergen P, De Lang H, Versteeg DHG. A hypotensive and bradycardic action of γ2-melanocyte stimulating hormone (γ2-MSH) microinjected into the nucleus tractus solitarii of the rat. Naun. Schmied. Arch. Pharmacol. 1994;349:50–56. doi: 10.1007/BF00178205. [DOI] [PubMed] [Google Scholar]
- 42.Li S-J, et al. Melanocortin antagonists define two distinct pathways of cardiovascular control by α- and γ-melanocyte stimulating hormones. J. Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkes NM, Watkin S, Stewart RD, Yen SSC. Localization and quantitation of β-endorphin in human brain and pituitary. Neuroendocrinology. 1980;30:113–121. doi: 10.1159/000122985. [DOI] [PubMed] [Google Scholar]
- 44.Ali-Rachedi A, et al. Immunocytochemical evidence for the presence of γ-MSH-like immunoreactivity in pituitary corticotrophs and ACTH-producing tumors. Neuroendocrinology. 1983;37:427–433. doi: 10.1159/000123588. [DOI] [PubMed] [Google Scholar]
- 45.Franco-Saenz R, Mulrow PJ, Kim K. Idiopathic aldosteronism: a possible disease of the intermediate lobe of the pituitary. J. Am. Med. Assoc. 1984;251:2555–2558. doi: 10.1001/jama.251.19.2555. [DOI] [PubMed] [Google Scholar]
- 46.Griffing GT, et al. Plasma immunoreactive gamma melanotropin in patients with idiopathic hyperaldosteronism, aldosterone-producing adenomas, and essential hypertension. J. Clin. Invest. 1985;76:163–169. doi: 10.1172/JCI111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekman R, Bjartell A, Lisander B, Edvinsson L. γ2-MSH immunoreactivity in the human heart. Life Sci. 1989;45:787–792. doi: 10.1016/0024-3205(89)90171-9. [DOI] [PubMed] [Google Scholar]