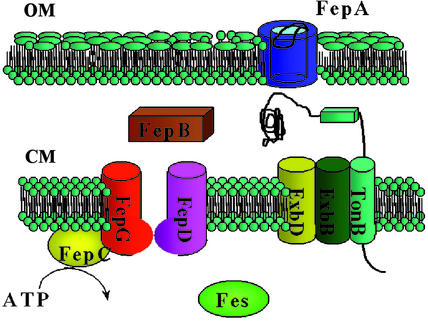
Figure 2.
A pictorial scheme shows the transmembrane topology of the FeEnt uptake proteins and how they function. In an iron-deficient state, iron receptors proliferate among the outer membrane (OM) proteins. FepA is a channel protein composed of a β-barrel and an N-terminal gate protein (see Fig. 3). The FepA receptor is highly specific and recognizes the iron binding domain and amide linkage domains of FeEnt. The gating movement of FepA is transduced by the complex TonB–ExbB–ExbD, which is anchored in the cytoplamic membrane (CM). FepB delivers FeEnt to the cytoplasmic pores formed by FepD and FepG. It appears that the cytoplasmic ATPase, FepC, provides energy to assist the uptake through the inner membrane. FeEnt esterase, which is encoded by the fes gene, catalyzes hydrolytic cleavage of the backbone, leading to the intracellular release of iron.