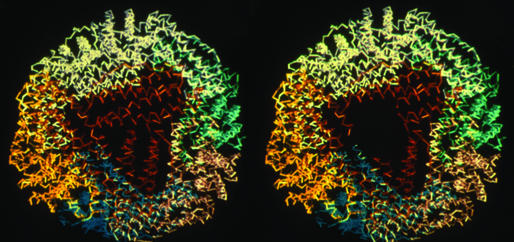
Figure 1.
Structure of a representative ferritin (recombinant frog L subunit) around one of the eight pores. Polypeptide backbones are colored to show the four-α-helix bundle subunits packed into the assembled 24-subunit structure, viewed down the threefold axis, at the pore. The structure, similar in >20 ferritin structures currently in the Protein Data Bank, is based on data from refs. 3 and 19. When the pore is open and the helices that form the pores are unfolded, as in recombinant frog H-L134P, the backbone structure remains superimposable on the WT frog L protein structure except at the pore, where no electron density was detectable in the x-ray diffraction pattern of protein crystals (3). (Right) The absence of pore structure is illustrated as the black or empty area, which was made from Left and the data in ref. 3.