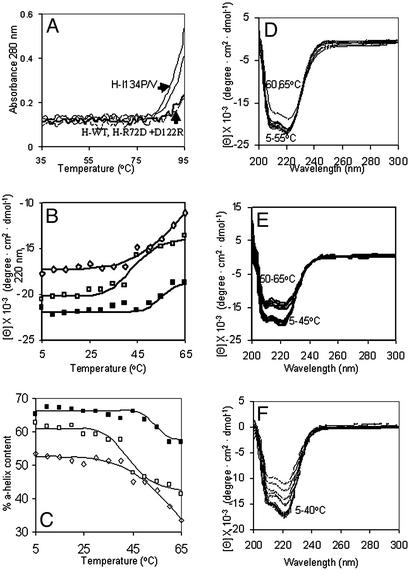
Figure 3.
Subdomain helix-reversible unfolding in the ferritin four-helix bundles. Solutions of recombinant ferritin (frog H-WT, H-L134P, H-R72D+, D122R, and H-WT + 1 mM urea) were analyzed in 0.01 M Mops, pH 7 (see Experimental Methods). CD spectra were recorded in a cuvette with a 1-cm path length at protein concentrations of 2 × 10−7 M except for H-L134P, the protein with fully open pores in protein crystals (Fig. 1), where the concentration was 4 × 10−7 M to compensate on the lower signal at higher temperatures. (A) UV-vis analysis of temperature transitions in global ferritin structure (A280) in WT and pore mutants (L134P, L134V, and R72D+D122R) between 35 and 95°C. (B) CD analysis of subdomain temperature transitions below global melting in ferritin between 5 and 65°C. ■, - H-WT; □, H-WT + 1 mM urea; ⋄, H-L134P. (C) Effect of temperature on % α-helix content in ferritin. ■, H-WT; □, H-WT + 1 mM urea; ⋄, H-L134P. (D–F) CD spectra of ferritin at different temperatures: D, H-WT; E, H-WT + 1 mM urea; F, H-L134P.