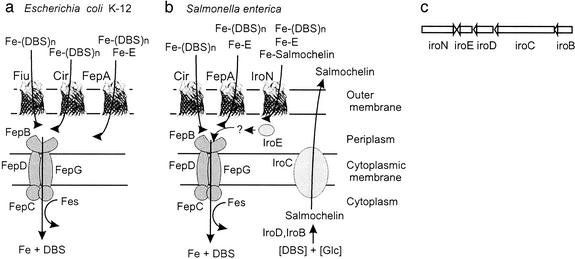
Figure 1.
Tentative scheme of the catecholate siderophore transport systems of E. coli K-12 (a) and S. enterica (b). In both systems, ferric enterochelin (Fe-E) is transported mainly through FepA across the outer membrane, whereas the linear degradation products, ferric DHBS (DHBS)n (n = 1, 2, 3), are taken up mainly via Fiu and FepA in E. coli. In Salmonella, this has not been studied in detail. However, because of the high similarity of the two systems, the same specificities can be expected. Iron complexes are then transported via the ABC transporter consisting of the binding protein FepB, the membrane components FepD and FepG, and the ATPase FepC through the cytoplasmic membrane. Inside the cell, the Fes protein is required for iron release from the ferric enterochelin complex. In Salmonella, salmochelins are synthesized from glucose and DHBS, possibly with the help of IroD and IroB and secreted by the exporter IroC. IroE might degrade the iron–salmochelin complexes to allow their transport via the ABC transporter FepBDGC. (c) The same gene order is found in the iroA gene locus of S. enterica and uropathogenic E. coli strains (5, 7).