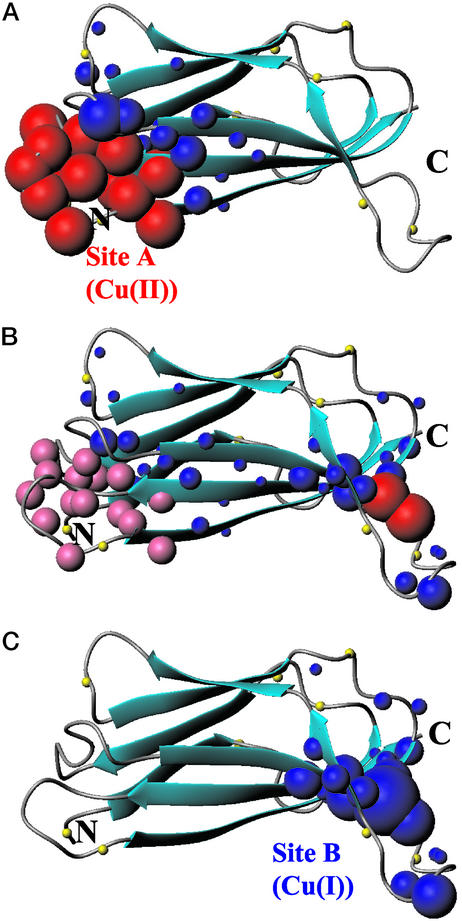
Figure 4.
Spectral changes occurring in 1H-15N HSQC spectra of apoCopC on addition of one equivalent of Cu(II) (A) and, on the latter adduct, of half (B) and one (C) equivalent of ascorbate are mapped on the apoCopC structure (9). Residues whose amide 1H-15N cross-peaks are broadened beyond detection are represented as red spheres. Pink spheres indicate residues whose signals experience slow exchange between the Cu(II) and the Cu(I) forms, giving rise to two sets with half intensity at 0.5 equivalent of ascorbate (B). Residues experiencing shift changes are shown as blue spheres whose radius is proportional to the shift change. The N atoms of proline residues are represented as yellow spheres.