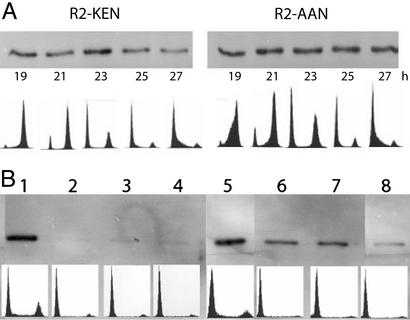
Figure 4.
Mutating the KEN signal stabilizes the R2 protein during mitosis and G1. (A) Stably transformed mouse Balb/3T3 fibroblasts overexpressing wild-type R2 protein (clone D2) or the AAN mutant R2 protein (clone MK6) were explanted on 5-cm dishes (2 × 105 cells per dish) and allowed to grow for 24 h in DMEM containing 10% horse serum. Then the cells were synchronized in DMEM containing 1% horse serum for 45 h and finally released by the addition of DMEM containing 20% horse serum. At different time points after serum readdition (19–27 h), cells overexpressing wild-type R2 protein (lanes 1–5) or the AAN mutant R2 protein (lanes 6–10) were harvested for flow cytometry and immunoblotting. (B) Stably transformed cells overexpressing native or AAN-mutated R2 protein (clones D4 and MK 2, respectively) were explanted on 5-cm dishes and allowed to grow for 24 h in DMEM containing 10% horse serum. After harvesting four dishes for flow cytometry and immunoblotting, the medium was changed to DMEM containing 1% serum and cells were harvested as before after 24 and 36 h. Finally, the cells were released from starvation with DMEM containing 20% serum and harvested 4 h after serum readdition. To make sure that the same amount of protein (0.3 μg) was loaded in each lane, the protein concentration of each cell extract was determined by the Bio-Rad protein assay before loading. Lane 1, logarithmically growing, wild-type R2 protein-overexpressing cells; lane 2, the same after 24 h of serum starvation; lane 3, the same after 36 h of serum starvation; lane 4, the same 4 h after serum readdition; lane 5, logarithmically growing, AAN mutant R2 protein-overexpressing cells; lane 6, the same after 24 h of serum starvation; lane 7, the same after 36 h of serum starvation; lane 8, the same 4 h after serum readdition. In both A and B, Lower shows the flow cytometry profile corresponding to each time point.