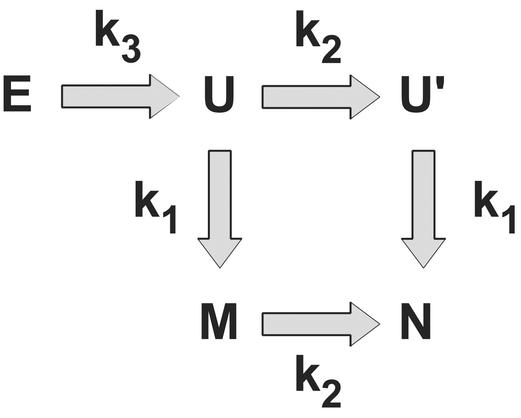
Figure 4.
The kinetic mechanism describing the folding of the FBP WW domain. E represents unfolded states in which loop 2 is extended, U represents the state in which loop 2 is collapsed but not native-like, and the remainder of the protein is unformed. U′ represents the state in which loop 2 is fully native-like, and the remainder of the protein is unformed. M represents the state in which loop 2 is misregistered, and the remainder of the protein is fully native-like. N represents the native state, in which the complete protein is formed. The observed rate constants (kobs = kfor + krev) are k1 = 3.9 × 10−5 τ−1, k2 = 5.8 × 10−6 τ−1, and k3 = 1.7 × 10−4 τ−1, where τ is the fundamental time scale.