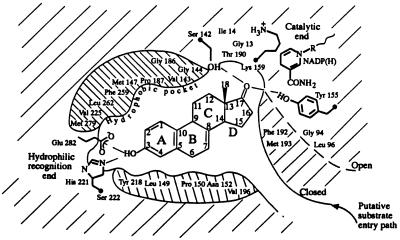
Figure 2.
A schematic diagram of the ligand-binding pocket (viewed roughly perpendicular to the β face of the equilin molecule) derived from the description of the active site provided in the text. The hydrophobic environment depicted above and below the steroid molecule is, in fact, comprised of residues that surround the ligand in the pocket. The open conformation of the substrate-entry path is shown in dashed line. In the ternary complex representing the closed conformation of the entry path, shown in solid line, residues Phe-192 and Met-193 form hydrophobic contacts with the ligand.