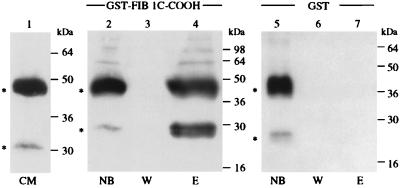
Figure 2.
The recombinant NOVH proteins produced by baculovirus-infected cells physically interacts with fib1C-COOH. Three hundred microliters of a 50% GST–fib1C-COOH Sepharose bead slurry (corresponding to 100 μg of recombinant GST–fib1C-COOH protein) were incubated for 14 hr at 4°C with 4 ml of conditioned medium from Sf9/82 cells expressing both the 44-kDa and 25-kDa NOVH proteins (see text). After washing the beads 5 times with 1 ml of lysis buffer, the proteins bound to the column were eluted with 40 μl of sample buffer and analyzed by using SDS/PAGE. The same procedure was used with Sepharose beads coupled to GST alone as a control for nonspecific binding. After protein transfer onto poly(vinylidene) difluoride membranes, the blots were reacted with the K19M NOVH-specific antibody as described (18). CM, serum-free conditioned medium from Sf9/82 cells; NB, proteins not bound to the columns; W, content of the last wash; E, proteins eluted from the columns.