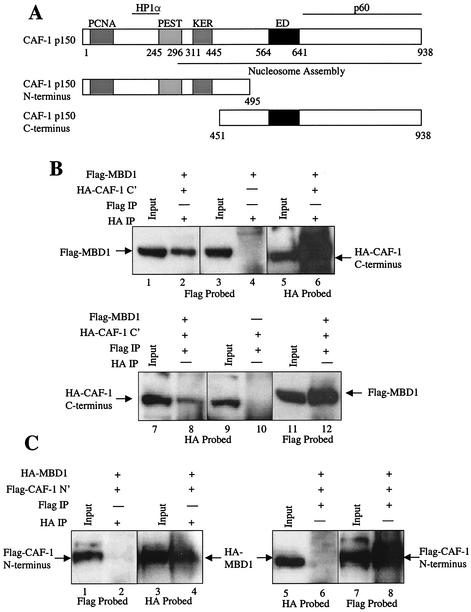
FIG. 3.
MBD1 associates with the C terminus of CAF-1 p150. (A) Map of CAF-1 p150 and C-terminal and N-terminal truncations. CAF-1 p150 contains N-terminal PCNA- and HP1α-interacting domains and a C-terminal CAF-1 p60 subunit-interacting domain. A large region of the protein is associated with nucleosomal assembly activity. An N-terminal fragment of CAF-1 p150 was created which lacks the C-terminal p60 binding domain, the ED motif, and the majority of the nucleosomal assembly domain. The C-terminal fragment lacks the PCNA and HP1α binding sites and the PEST and KER domains. (B) MBD1 and the C terminus of CAF-1 p150 coimmunoprecipitate in mammalian cells. Protein extracts from COS7 cells coexpressing both Flag-MBD1 and HA-CAF-1 p150 C terminus were immunoprecipitated (IP) with (+) an anti-HA MAb (lanes 2, 4, and 6) and an anti-Flag MAb (lanes 8, 10, and 12). Extracts from cells expressing Flag-MBD1 alone and HA-CAF-1 p150 C terminus alone were immunoprecipitated with the anti-HA MAb (lane 4) and anti-Flag MAb (lane 10), respectively. Western blots were probed with the anti-Flag MAb (lanes 1 to 4 and 11 and 12) and the anti-HA MAb (lanes 5 and 6 and 7 to 10). The input represents 10% of the total protein extract used for the immunoprecipitations. (C) MBD1 does not complex with the N terminus of CAF-1 p150 in mammalian cells. COS7 cells were cotransfected with HA-MBD1 and Flag-CAF-1 p150 N terminus. Extracts from these cells were immunoprecipitated with an anti-HA MAb (lanes 2 and 4) and an anti-Flag MAb (lanes 6 and 8). Western blots were probed with the anti-Flag (lanes 1 and 2 and 7 and 8) and anti-HA (lanes 3 to 6) MAbs. Ten percent of the total extract used in the immunoprecipitations was used as the input.