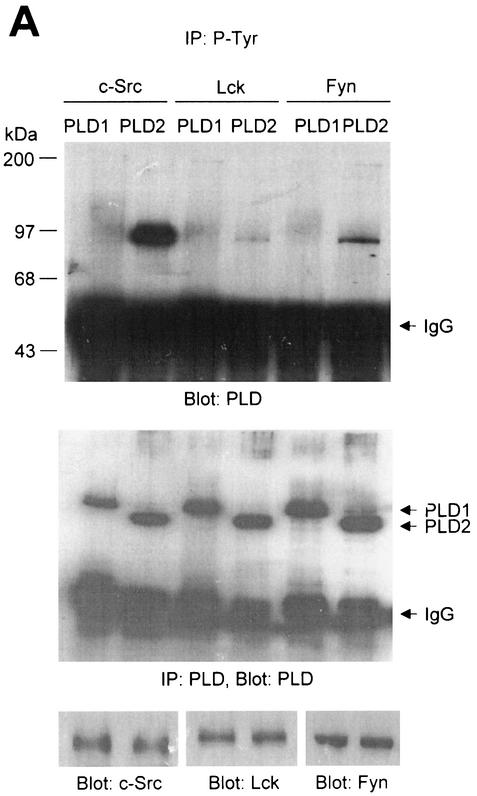
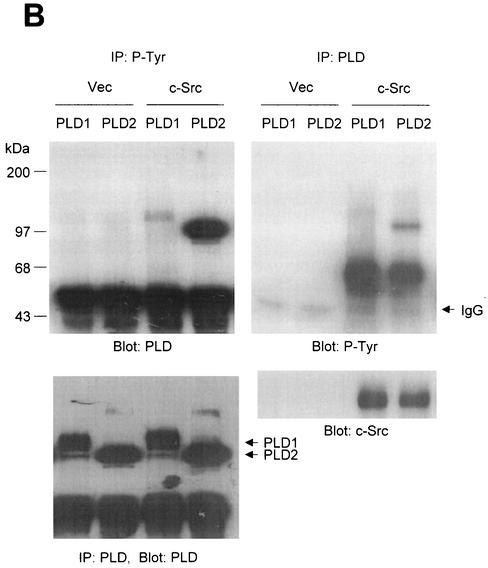
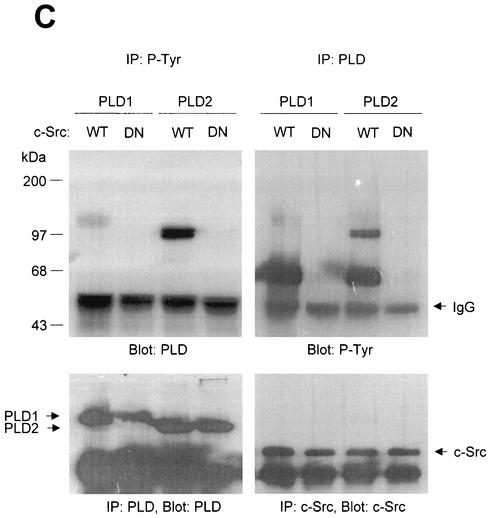
FIG. 1.
PLD2 and, to a lesser extent, PLD1 are tyrosine phosphorylated by c-Src tyrosine kinase. (A) COS-7 cells were transiently cotransfected for 40 h with various combinations of plasmids encoding PLD1, PLD2, c-Src, Lck, and Fyn. Cell lysates were immunoprecipitated with anti-P-Tyr antibody, and precipitated proteins were subjected to immunoblot analysis with an anti-PLD antibody. Western blot analysis using PLDs and c-Src family tyrosine kinase demonstrated that the respective proteins were expressed to equal extents. (B) COS-7 cells were transiently transfected with the indicated expression plasmids, and cell lysates were subjected to immunoprecipitation with anti-P-Tyr antibody or anti-PLD antibodies. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-PLD or anti-P-Tyr antibody. Expression of PLD and c-Src was determined by using anti-PLD or anti-c-Src antibodies. (C) COS-7 cells were transiently transfected with various combinations of plasmids encoding PLD1, PLD2, wild-type (WT) c-Src, and dominant negative (DN) c-Src. Cell lysates were immunoprecipitated by using anti-P-Tyr antibody or anti-PLD antibody, and immune complexes were analyzed by immunoblotting. Immunoreactive proteins were visualized by use of horseradish peroxidase-coupled secondary antibody and chemiluminescence. Data are representative of three experiments.