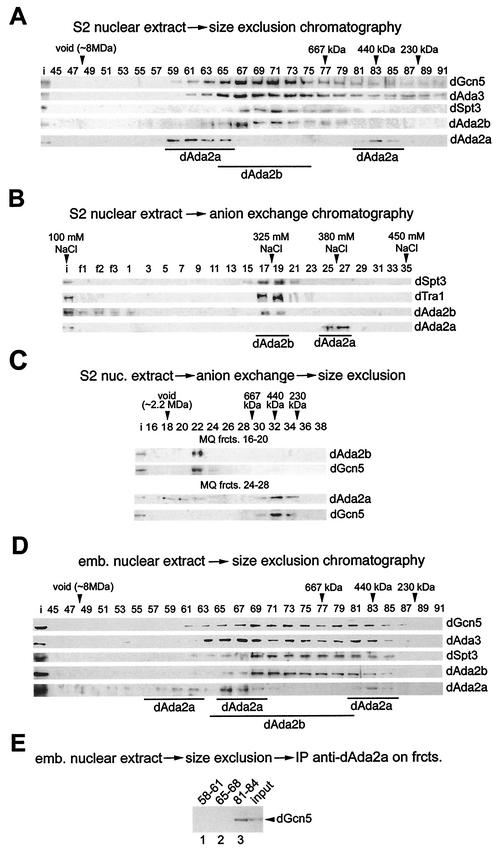
FIG. 4.
dAda2b associates with dSAGA-specific subunits in biochemical fractionation assays. (A) Elution profiles of dGcn5, dAda3, dSpt3, dAda2b, and dAda2a from a Sephacryl S400 gel filtration column; 5 mg of S2 nuclear extract was separated on the column, and 15 μl of each fraction was subjected to SDS-PAGE, followed by Western blotting (fraction numbers indicated at the top). dGcn5 and dAda3 coelute in a range from ∼2.2 MDa to 300 kDa. dAda2b coelutes with dSpt3 in a range of ∼2 MDa to 800 kDa. dAda2a elutes in two peaks around 2.5 MDa and 440 kDa. i, input (30 μg of embryonic nuclear extract). (B) dSpt3, and dTra1 cofractionate with dAda2b, but not dAda2a, in anion-exchange chromatography. Bound proteins were eluted in a linear gradient of 100 to 450 mM sodium chloride from a 1-ml MonoQ anion-exchange column. The salt concentrations of the peak fractions are indicated above the blot. Fifteen microliters of each fraction was subjected to SDS-PAGE, followed by Western blotting (fraction numbers indicated at the top; f1 to f3, flowthrough). (C) MonoQ (MQ) fractions (frcts.) 16 to 20 (top) and 24 to 28 (bottom) of the anion-exchange chromatography were concentrated and subjected to size exclusion chromatography. dAda2b and dGcn5 coelute in a range of ∼1.8 MDa. dAda2a elutes in fractions of >2 MDa and ∼450 kDa. dGcn5 is detectable only in fractions containing the smaller dAda2a complex. nuc., nuclear. (D) Gel filtration analysis of nuclear extract from 0- to 12-h-old embryos as described for panel A. A third dAda2a peak is observed in a molecular-mass range of ∼1.8 MDa. (E) Pooled dAda2a peak fractions from embryonic extracts (panel D) were subjected to immunoprecipitation assays with affinity-purified anti-dAda2a antibodies. The immunoprecipitates (IP) were separated by SDS-PAGE and were probed for dGcn5. Lane 1, fractions 58 to 61; lane 2, fractions 65 to 68; lane 3, fractions 81 to 84.