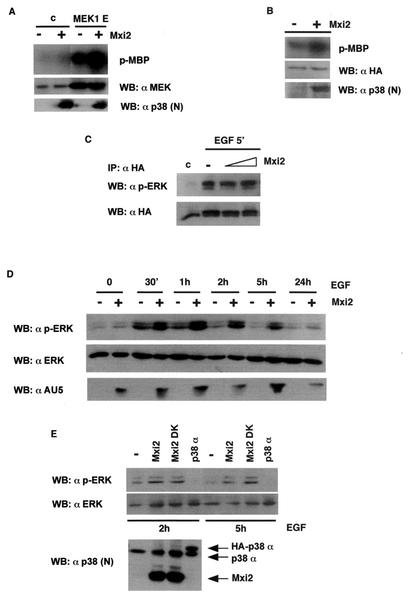
FIG. 6.
Effects of Mxi2 on ERK phosphorylation and activation levels. (A) Effects of Mxi2 on the activation of ERK2 by MEK. 293T cells were transfected with HA-ERK2 cotransfected with vector or with MEK1E (1 μg) in the presence (+) or absence (−) of Mxi2 (1 μg). Phosphorylated MBP (p-MBP) levels indicate ERK activity levels. (Bottom) Expression levels of MEK and Mxi2. (B) Effect of Mxi2 on ERK2 basal activity levels. Cells were transfected with HA-ERK2 (1 μg) in the presence (+) or absence (−) of Mxi2 (1 μg). After 18 h of starvation, ERK kinase activity was determined as described previously. Phospho-MBP levels became apparent after prolonged exposure at −70°C. (Bottom) Expression levels of HA-ERK2 and Mxi2. (C) Effects of Mxi2 on EGF-induced phosphorylation of ERK2. Cells transfected with HA-ERK2 (1 μg) and increasing amounts of Mxi2 (2 and 5 μg) were stimulated with EGF for 5 min and probed for phospho-ERK in anti-HA immunoprecipitates. (Bottom) Levels of immunoprecipitated HA-ERK2. (D) Effects of Mxi2 on the dephosphorylation rate of ERK2. Cells were transfected with (+) or without (−) AU5-Mxi2 and stimulated with EGF (100 ng/ml), and phospho-ERK levels in total lysates were examined at the indicated times. (Bottom) Expression of ERK1/2 and AU5-Mxi2. (E) Effects of Mxi2 dead kinase (DK) on ERK2 dephosphorylation. Cells transfected with the indicated constructs were stimulated with EGF, and phospho-ERK levels were determined after 2 and 5 h. (Bottom) Expression of p38 proteins detected by immunoblotting with anti-p38 N terminus antibody. WB, Western blot; c, control.