FIG. 7.
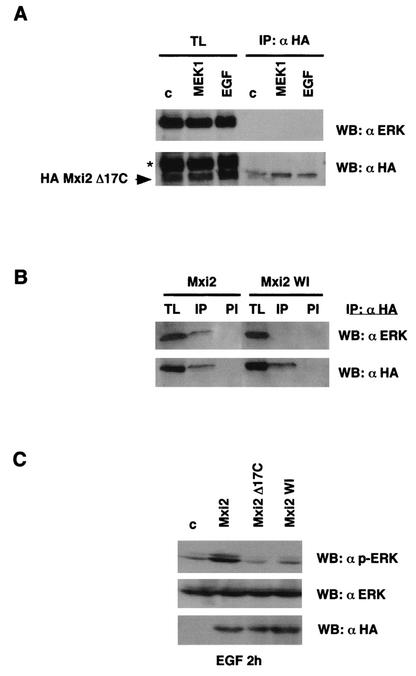
Effects of Mxi2 on ERKs require the C terminus of Mxi2. (A) ERKs cannot bind to a deletion mutant form of Mxi2 lacking the C terminus. 293T cells were transfected with HA-Mxi2 Δ17C (1 μg) and stimulated with EGF (100 ng/ml) for 5 min or with cotransfected MEK1 E where indicated. Anti-HA immunoprecipitates (IP) and their respective total lysates (TL) were probed for the presence of ERK. (Bottom) Levels of HA-Mxi2 Δ17C protein. The position of HA-Mxi2 Δ17C is indicated. The asterisk shows the remaining anti-ERK signal from the previous blot. (B) ERKs cannot bind to the Mxi2 WI mutant form. Cells were transfected with HA-Mxi2 or with HA-Mxi2 WI (1 μg). Lysates were immunoprecipitated with an anti-HA antibody or with preimmune (PI) serum and probed for associated ERK. (Bottom) Expression of the HA-tagged proteins. (C) Effect of the mutant form of Mxi2 lacking the C terminus on ERK2 dephosphorylation. Cells transfected with the indicated constructs (1 μg) were stimulated with EGF (100 ng/ml), and phospho-ERK levels were determined after 2 h. (Bottom) Total ERK protein levels and expression of the Mxi2 HA-tagged proteins detected by anti-ERK and anti-HA immunoblotting, respectively. WB, Western blot; c, control.