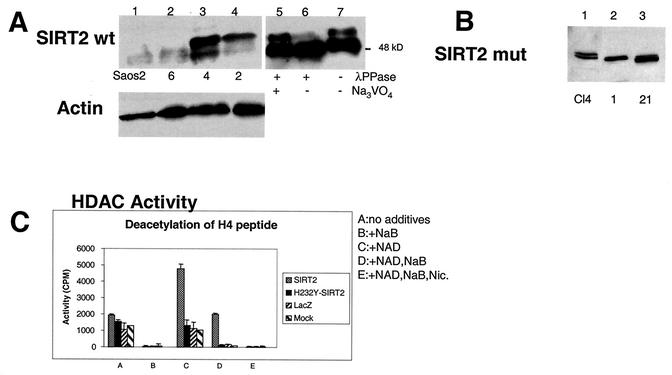
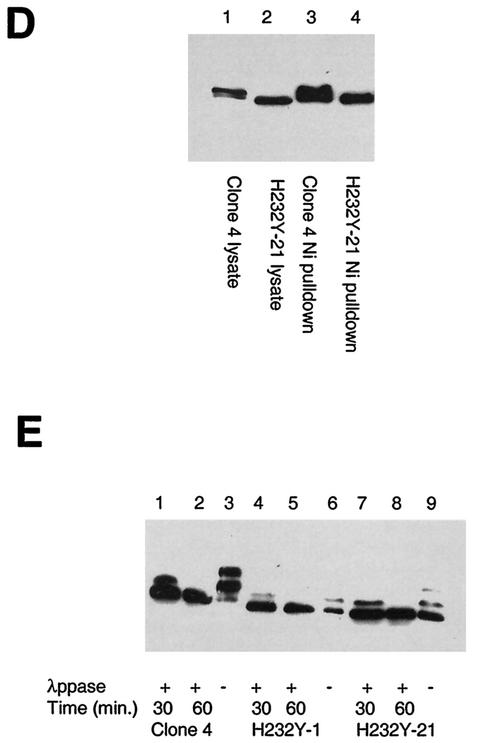
FIG. 3.
SIRT2 expression in stable transfectants of Saos2 cells. Saos2 cells stably transfected with pcDNA3.1-SIRT2 or pcDNA3.1-SIRT2 H232Y express various levels of the phosphorylated form of SIRT2. Crude lysates from individual clones were subjected to immunoblotting with the SIRT2 peptide antibody. (A) Stable transfectants expressing wild-type (wt) SIRT2. Cells ± λPPase and Na3VO4 were used as markers for phosphorylation status. Lanes: 1, untransfected Saos2 cells; 2, clone 6; 3, clone 4; 4, clone 2. Lanes 5 to 7 contained nocodazole-treated cells ± λPPase and Na3VO4. To control for protein loading, blots were also probed with actin (lower panel). (B) Stable transfectants expressing mutant SIRT2, in which histidine 232 was mutated to tyrosine. Lane 1 contained clone 4. Clones 1 and 21 (lanes 2 and 3, respectively) were used in subsequent experiments. (C) SIRT2 NDAC activity in various transfected Saos2 cell lines. Activity was assayed by monitoring the release of 3H-acetyl groups from an H4 peptide (counts per minute) under the conditions indicated. (D) The amino- and carboxy-terminal domains of the H232Y clones are intact. Nickel magnetic agarose beads were used to purify SIRT2. Crude cell lysates prior to pulldown assays are shown in lanes 1 and 2. Lysates and pulldown assays were subjected to immunoblotting with the SIRT2 peptide antibody. (E) Time course of λPPase digestion of SIRT2 stable transfectants. The hyperphosphorylated isoform appears to be the most labile, collapsing sequentially to the final hypophosphorylated SIRT2 isoform.