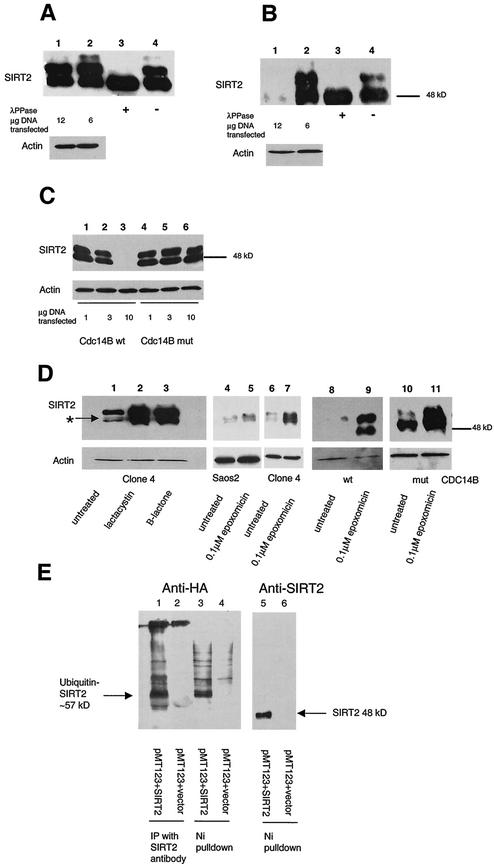
FIG.5.
Transfection of functional Cdc14B into clone 4 causes SIRT2 protein levels to decrease and targets SIRT2 for ubiquitination and subsequent degradation by the 26S proteasome. Clone 4 cells were transiently transfected with CDC14A, CDC14B, or the CDC14B C314S mutant at the indicated DNA concentrations. As a control, a lysate from untransfected clone 4 cells was treated with or without λPPase (lanes 3 and 4, respectively, in panels A and B). Ten micrograms of crude protein lysate was loaded in each lane and subjected to immunoblotting with the SIRT2 peptide antibody. (A) Clone 4 transfected with CDC14A. There was very little change in SIRT2 when either 6 or 12 μg of CDC14A DNA was transfected into cells (lanes 1 and 2). The arrow marked with an asterisk indicates the unphosphorylated SIRT2 isoforms. (B) Clone 4 transfected with CDC14B. When 12 μg of CDC14B was transfected into cells, there was a dramatic decrease in protein and loss of the phosphorylated SIRT2 (lane 1). (C) Clone 4 transfected with the either CDC14B wild type (wt) or a Cdc14B C314S mutant (mut) that has no phosphatase activity. With increasing amounts of CDC14B, there was a corresponding decrease in SIRT2 (lanes 1 to 3). There was no change in SIRT2 with increasing concentrations of the CDC14B C314S mutant plasmid (lanes 4 to 6). (D) The 26S proteasome inhibitors lactacystin, clasto-lactacystin β-lactone, and epoxomicin stabilize SIRT2 isoforms and prevent ubiquitin-mediated degradation. Following a 20-h incubation with the indicated inhibitors, cells were harvested and processed for Western blot analysis. Lanes: 1 and 6, untreated clone 4 lysates; 2, clone 4 treated with 10 μM lactacystin; 3, clone 4 treated with 10 μM clasto-lactacystin β-lactone; 4, untreated Saos2 lysate; 5, Saos2 treated with 0.1 μM epoxomicin; 7, clone 4 treated with 0.1 μM epoxomicin; 8 and 9, clone 4 transfected with CDC14B (wt) minus or plus 0.1 μM epoxomicin, respectively; 10 and 11, clone 4 transfected with CDC14B C314S (mut) minus or plus 0.1 μM epoxomicin, respectively. To control for protein loading, all blots were probed with actin as well. (E) SIRT2 and ubiquitin coprecipitate in both nickel magnetic agarose bead pulldown assays and protein A SIRT2 immunoprecipitations. Saos2 cells were transiently cotransfected with the HA-tagged, ubiquitin-expressing plasmid pMT123 and either pcDNA3.1-SIRT2 or the empty vector pcDNA3.1/HisC. Lysates from the transfected cells were incubated with Ni-NTA magnetic agarose beads or SIRT2 antibody bound to protein A affinity gel. Samples were processed as described above and immunoblotted with HA-ll antibody (lanes 1 to 4) or SIRT2 peptide antibody (lanes 5 and 6).