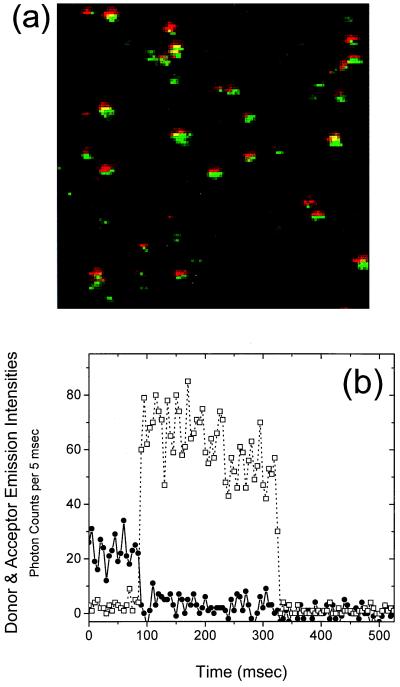
Figure 1.
(a) Dual-color composite image of doubly labeled SNase molecules immobilized by means of histidine tag in buffer A at 23°C. The 514-nm excitation laser spot (Ar+ light, 15 μW) was scanned from top to bottom and left to right. Each spot represents a single protein. Donor emission is colored green and acceptor emission is colored red. (b) Emission time trace of a single double-labeled SNase molecule. The resolution of the measurement is 5 msec. The instantaneous donor and acceptor emission intensities, Id and Ia (squares and circles, respectively), are related to the energy transfer efficiency by the expression E(t) = [1 + γId/Ia]−1, where γ is a correction factor determined to be 0.8. The SNase molecule shown here displays a very high degree of energy transfer (high acceptor intensity, low donor intensity) from 0 to 80 msec. At 80 msec, photodestruction of the acceptor occurs and donor emission simultaneously increases. The inverse correlation is direct evidence for spFRET.