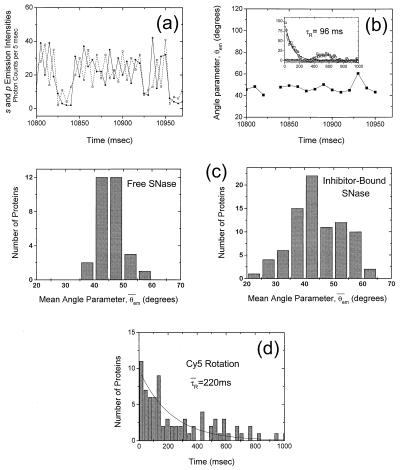
Figure 3.
(a) A typical smFPA time trace of Cys28 TMR-labeled SNase immobilized by means of histidine tag in buffer A. The two orthogonally polarized emissions, Is (dotted) and Ip (solid), are displayed as a function of time. Because correlated emissions correspond to a fixed fluorophore dipole and anticorrelated emissions correspond to a rapidly rotating fluorophore dipole, the SNase-conjugated TMR molecule shown here is rotating rapidly (much faster than the data integration time of 5 msec). (b) The angle parameter θem(t) calculated from a. The value of θem(t) is close to 45° when the fluorophore is rotating rapidly with little restriction (14). The break in graph represents a dark-state transition. In the inset is the autocorrelation of the angle parameter (in circles); there are clearly no significant temporal fluctuations in TMR rotation on the millisecond time scale. Also in the inset is the angle parameter autocorrelation for pTp-bound Cys28 TMR-labeled SNase (squares) together with an exponential fit. Here the rotational fluctuations are substantial, with a characteristic time constant τR of 96 msec. (c) Histograms of θ̄ for immobilized TMR-labeled SNase molecules with and without inhibitor pTp. The distribution is narrowly centered at 45° for uninhibited SNase, indicative of free and rapid rotation of the attached TMR fluorophore. The TMR of inhibitor-bound SNase, on the other hand, displays hindered and fluctuating rotational behavior, indicated by the broader mean angle parameter histogram; (d) Histogram of rotational fluctuation time constants τR for Cy5-labeled SNase. Only those molecules that showed single-step photobleaching were included to screen out multiply Cy5-labeled cases. The average value of τR is 220 msec, considerably longer than the majority of E(t) fluctuation time constants (average τE = 41 msec, Fig. 2c) .