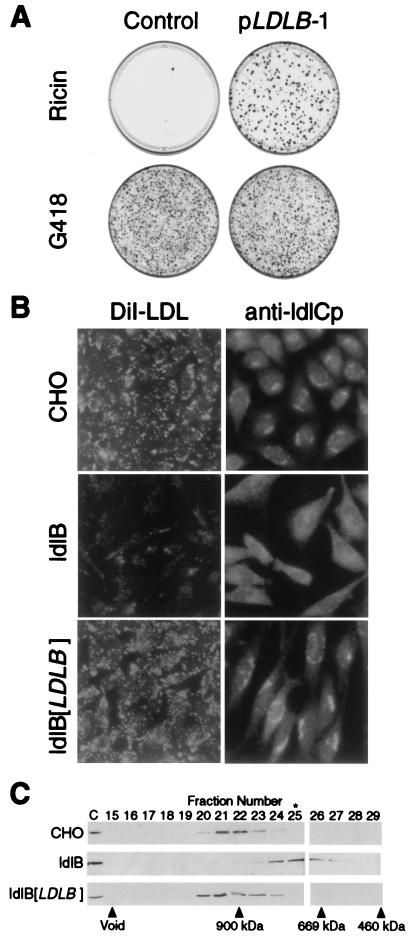
Figure 2.
LDLB cDNA corrects the mutant phenotypes of ldlB cells. (A) Ricin sensitivity. ldlB cells were transfected with either pLDLB-1 or pcDNA3.1 (control). Two days later, the cells were replated and selected with ricin (medium E) or G418 (medium I, transfection efficiency control). Twelve days later, the dishes were fixed and stained with crystal violet. (B) LDLR activity and Golgi localization of ldlCp. Left, wild-type CHO, ldlB, and ldlB[LDLB] cells were grown on glass cover slips in medium F, and LDLR activity, as measured by uptake of fluorescent DiI-LDL (1 μg protein per ml, 1 hr), was observed by using a ×40 objective lens as described (see Materials and Methods). Right, Wild-type CHO, ldlB, and ldlB[LDLB] cells were grown on glass cover slips in medium B and immunostained with a polyclonal anti-ldlCp antibody followed by FITC-labeled goat anti-rabbit IgG and observed by using a ×63 objective lens as described (see Materials and Methods). Golgi localization of ldlCp in CHO and ldlB[LDLB] cells was confirmed by double staining with anti-β-COP antibody (not shown). (C) Gel chromatographic analysis of ldlCp. Cytosol was prepared from wild-type CHO, ldlB, and ldlB[LDLB] cells and analyzed by Superose 6 chromatography as described (see Materials and Methods). Samples were subjected to SDS/PAGE and immunoblotting with affinity-purified polyclonal anti-ldlCp antibody. Fractions containing the ≈80-kDa ldlCp band, as well as unfractionated, diluted (1:10) cytosol (C) are shown. The column was calibrated with Mr standards ranging from 65 to 900 kDa, including apoferritin (460 kDa), thyroglobulin (669 kDa), and IgM (900 kDa) (vertical arrows). The peak fraction of β-COP-containing coatomer, determined by immunoblotting with a monoclonal antibody against β-COP (not shown), is indicated by ∗.