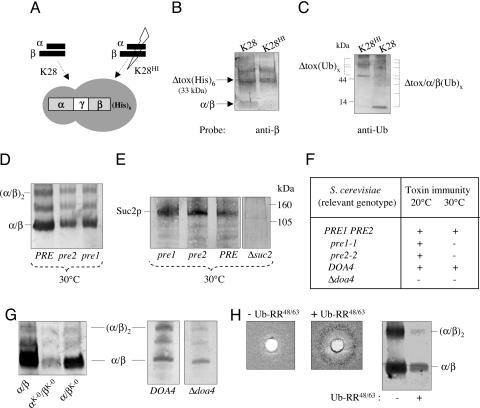
Fig. 3.
Functional immunity requires ubiquitination and proteosomal degradation of the pptox/K28 complex. (A) Schematic drawing of a His-tagged K28 pptox derivative lacking its N-terminal prepro sequence [Δtox(His)6] and experimental setup to copurify a Δtox(His)6/toxin complex in vivo. Yeast transformants expressing the His-tagged K28 toxin precursor Δtox(His)6 were spheroplasted and treated with either native toxin (K28) or heat-inactivated toxin (K28HI), the latter being completely blocked in cell entry. Cells were lysed, cell debris and membranes were removed, and the resultant supernatant applied onto a TALON column to purify the His-tagged K28-derivative. (B) Demonstration of a Δtox(His)6/α/β complex purified from the cytosol of toxin-immune cells. After purification, eluted proteins from A were fractionated by SDS/PAGE and probed with polyclonal anti-β subunit antibody. Positions of the His-tagged protoxin [Δtox(His)6] and the heterodimeric α/β toxin are indicated. (C) Evidence that the Δtox(His)6/α/β complex is ubiquitinated and proteasomally degraded. Eluted protein samples from A were fractionated by SDS/PAGE and detected by using polyclonal anti-Ub antibody. Positions of the ubiquitinated toxin precursor [Δtox(Ub)x] and the breakdown products of the ubiquitinated Δtox/α/β(Ub)x complex are indicated. (D) Western blot analysis of secreted K28 toxin and (E) invertase Suc2p in cell-free culture supernatants. (F) Impaired toxin immunity in conditional proteasome mutants pre1–1 and pre2–2 and a Δdoa4 knockout compared with wild-type. As negative control for invertase secretion, the Δsuc2 mutant S. cerevisiae SEY6210 was used. (G) Relative amount of secreted K28 wild-type toxin (α/β) and its two lysine-free variants α/βK-0 and αK-0/βK-0 in S. cerevisiae SEY6210 and decreased α/β toxin secretion in a yeast Δdoa4 mutant defective in protein deubiquitination compared with the isogenic DOA4 wild type. Positions of the α/β heterodimeric toxin and its tetrameric deriative (α/β)2 are indicated. (H) Lack of functional immunity and decrease in toxin secretion in K28 pptox-expressing wild-type cells before and after Cu2+-induced overexpression of mutated Ub (Ub-RR48/63) incapable of forming polyubiquitin chains.