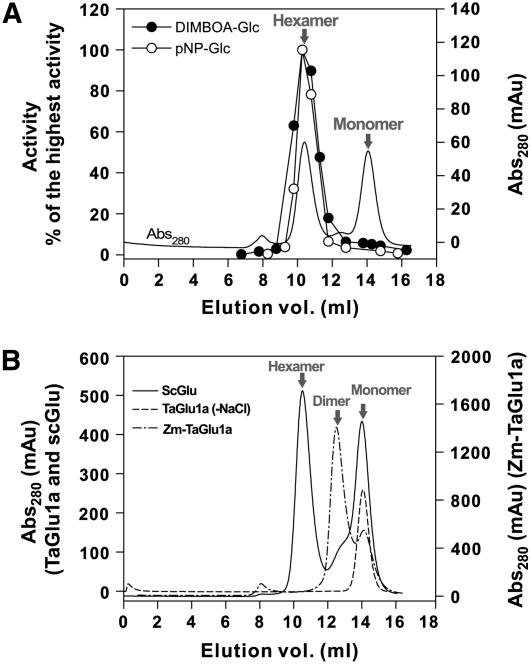
Figure 4.
Gel filtration of TaGlu1a, ScGlu, and Zm-Glu1a. A, The enzyme solution eluted from the nickel column was further purified by gel filtration using a Superdex 200 column. Each fraction volume was 0.5 mL. The eluted protein was monitored by A280. The two protein peaks correspond to hexamer and monomer. B, Solid line, Gel filtration of ScGlu was performed after affinity chromatography. Two peaks of hexamer and monomer were observed. Dashed line, TaGlu1a (wild type) purified by affinity chromatography followed by gel filtration was dialyzed against HEPES buffer without NaCl and subjected to gel filtration analysis. Only a monomeric protein was detected. Dash-dot line, Zm-TaGlu1a purified by affinity chromatography was further purified by gel filtration. While the hexamer peak was not detected, the dimer and monomer peaks were observed.