Abstract
Using the short-lived radiotracer 42K+, we present a comprehensive subcellular flux analysis of low-affinity K+ transport in plants. We overturn the paradigm of cytosolic K+ pool-size homeostasis and demonstrate that low-affinity K+ transport is characterized by futile cycling of K+ at the plasma membrane. Using two methods of compartmental analysis in intact seedlings of barley (Hordeum vulgare L. cv Klondike), we present data for steady-state unidirectional influx, efflux, net flux, cytosolic pool size, and exchange kinetics, and show that, with increasing external [K+] ([K+]ext), both influx and efflux increase dramatically, and that the ratio of efflux to influx exceeds 70% at [K+]ext ≥ 20 mm. Increasing [K+]ext, furthermore, leads to a shortening of the half-time for cytosolic K+ exchange, to values 2 to 3 times lower than are characteristic of high-affinity transport. Cytosolic K+ concentrations are shown to vary between 40 and 200 mm, depending on [K+]ext, on nitrogen treatment (NO3− or NH4+), and on the dominant mode of transport (high- or low-affinity transport), illustrating the dynamic nature of the cytosolic K+ pool, rather than its homeostatic maintenance. Based on measurements of trans-plasma membrane electrical potential, estimates of cytosolic K+ pool size, and the magnitude of unidirectional K+ fluxes, we describe efflux as the most energetically demanding of the cellular K+ fluxes that constitute low-affinity transport.
Primary potassium uptake by plants has been described as the sum of activities of two distinct membrane transport systems (Epstein et al., 1963; Kochian and Lucas, 1982; Hirsch et al., 1998). The high-affinity transport system (HATS) operates primarily at low external concentrations (<1 mm) of K+ and catalyzes an inward flux, against an electrochemical gradient, by use of a K+/H+ symport mechanism (Véry and Sentenac, 2003). The low-affinity transport system (LATS), by contrast, dominates at higher external concentrations (>1 mm), mostly via the activity of potassium channels (Maathuis and Sanders, 1997). The distinctive characteristics of these systems are adaptive to highly variable soil K+ concentrations (Ashley et al., 2006), which typically range from 0.1 to 6 mm (Adams, 1971), and are often found at much higher values (Reisenauer, 1966), especially after fertilizer application. Thus, plant roots engage HATS and LATS transporters to varying degrees (Kochian and Lucas, 1982; Ashley et al., 2006). It is important to study the physiology of these systems because the differential sensitivities of high- and low-affinity transporters to environmental stressors, such as sodium (Na+) or ammonium (NH4+), can have profound influences on plant survival in the field. For instance, NH4+ suppresses high-affinity K+ transport (Scherer et al., 1984; Vale et al., 1987, 1988; Morgan and Jackson, 1984; Hirsch et al., 1998; Spalding et al., 1999; Santa-Maria et al., 2000; Ashley et al., 2006), while low-affinity K+ transport is relatively NH4+ insensitive and contributes to the relief from NH4+ toxicity at high K+ (Santa-Maria et al., 2000; Britto and Kronzucker, 2002; Kronzucker et al., 2003b). Similarly, variable sensitivities of K+ transporters to soil Na+ are critical factors in salinity tolerance (Epstein et al., 1963; Kochian et al., 1985; Tester and Davenport, 2003; Volkov et al., 2004; Kader and Lindberg, 2005).
Here, we demonstrate that unidirectional K+ influx systems are only one aspect of a more comprehensive physiological condition or “transport mode,” in which changes from a high- to a low-affinity mode are linked to major shifts in the plant's cellular ion relations. Some literature reports indicate that the low-affinity mode may display increased rates of both efflux (Pettersson and Kasimir-Klemedtsson, 1990; Kronzucker et al., 2003b) and cytosolic K+ exchange (Pierce and Higinbotham, 1970; Kochian and Lucas, 1982; Kronzucker et al., 2003b), but these fundamental characteristics of K+ transport and compartmentation have, until now, not been comprehensively investigated. It is better established that the electrical potential difference (ΔΨ) across the plasma membrane of plant cells progressively depolarizes in response to increasing external [K+] ([K+]ext; Etherton and Higinbotham, 1960; Higinbotham et al., 1964; Pitman and Saddler, 1966; Cheeseman and Hanson, 1979; Beilby and Blatt, 1986; Newman et al., 1987; Kochian et al., 1989). This electrical effect is intimately related with the voltage sensitivity of inwardly and outwardly rectifying K+ channels (Czempinski et al., 1997; Tyerman and Skerrett, 1999; Zimmermann and Sentenac, 1999), and has consequences for the energetics of K+ transport.
It is furthermore not well understood how transport mode influences cytosolic potassium homeostasis. The cytosolic concentration of K+ ([K+]cyt) has been thought to be maintained stringently near 100 mm, via fluxes from potassium pools in the external medium and the vacuole (Leigh and Wyn Jones, 1984; Beilby and Blatt, 1986; Maathuis and Sanders, 1993; Walker et al., 1996; Leigh, 2001; Ashley et al., 2006). However, we have previously demonstrated (Kronzucker et al., 2003b) that [K+]cyt can be reduced significantly when plant roots are exposed to NH4+. Similarly, others have shown that Na+ stress can depress [K+]cyt (Jeschke and Stelter, 1976; Harvey et al., 1981; Mills et al., 1985; Hajibagheri et al., 1987, 1988, 1989; Flowers and Hajibagheri, 2001; Carden et al., 2003). In addition, our previous data (Kronzucker et al., 2003b) suggested that [K+]cyt may also, surprisingly, have an inverse relationship with [K+]ext under some conditions, such as when [K+]ext is increased from a HATS condition (0.1 mm) to a transitional condition between HATS and LATS (1.5 mm; see Kochian and Lucas, 1982; Kochian et al., 1985). These findings warranted a rigorous investigation of the following question: Are increases in [K+]ext in the LATS range universally associated with deflections in [K+]cyt, and, if so, what is the nature of these deflections?
In this study, we use a combination of tracer-flux and thermodynamic analyses to characterize these and other key aspects of the low-affinity K+ transport mode. We present results from two methods of subcellular flux analysis in intact barley seedlings with the short-lived radiotracer 42K+, both of which obviate the problems associated with the use of 86Rb+ as a nonisotopic tracer for potassium (see Jeschke, 1970) and with effects of tissue excision (Britto et al., 2006). Steady-state potassium influx, efflux, net flux, cytosolic exchange kinetics, and cytosolic concentrations of this essential nutrient ion are reported and examined in the context of a thermodynamic analysis that draws upon electrophysiological measurements and provides for the first time, to our knowledge, a quantitative appraisal of the energetic cost of unidirectional K+ efflux from the roots of plants. Our study puts forward strong evidence for the breakdown of cytosolic K+ homeostasis in response to high [K+]ext and the striking demonstration of rapid and futile cycling of potassium at the plasma membrane of plant cells—two characteristics that are shown to define the LATS condition.
RESULTS
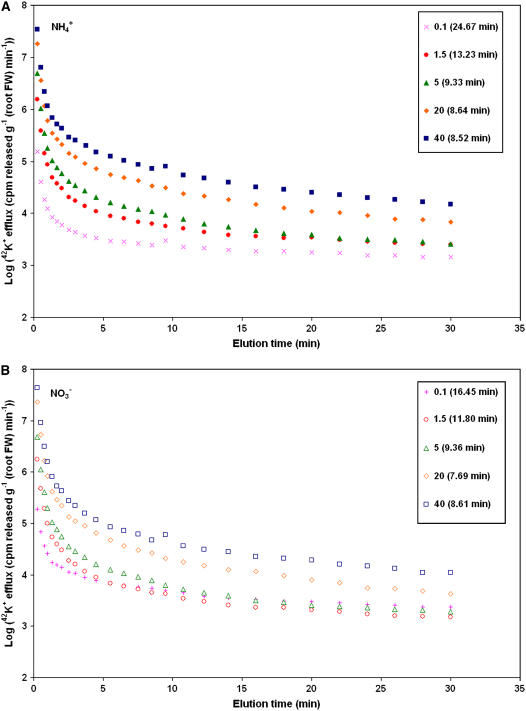
Figures 1 and 2 show results of the two methods (elution and subsampling) used to measure K+ efflux from barley roots that were grown, labeled, and desorbed at five steady-state external K+ concentrations (0.1–40 mm) and two nitrogen (N) sources (10 mm NH4+ or NO3−). Figure 1 shows the time dependence of 42K+ efflux from elution experiments, in the form of standard semilogarithmic plots of changing 42K+ release rates over time, resolved into three exponential phases of efflux (Siddiqi et al., 1991; Kronzucker et al., 1995). Linear regression of the slowest-exchanging of these phases, which represents tracer release from the cytosol (Memon et al., 1985a; Kronzucker et al., 2003b), revealed strongly variable patterns of efflux, depending on [K+]ext and N source. One aspect of this variability was the tendency for the half-time of cytosolic K+ exchange, as determined from the slopes of the regressed cytosolic lines, to decline with increasing K+ supply, ranging from 16 to 25 min in the HATS condition (0.1 mm [K+]ext) to shorter values in the LATS conditions, which cluster between 8 and 13 min. Secondly, the y intercepts of the cytosolic lines increased with increasing [K+]ext, indicating a greater degree of efflux as potassium supply goes up. The one exception to this was the slightly greater efflux seen at 0.1 mm K+ relative to 1.5 mm (a transitional concentration at the low end of the LATS range), in nitrate-grown plants.
Figure 1.
Comparison of 42K+ efflux patterns, as determined by tracer elution, in roots of barley seedlings grown under five K+ concentrations and two N treatments, NH4+ (A) and NO3− (B). Plots have been corrected for differences in root mass and tracer activity (to the arbitrary value of 2 × 105 cpm μmol−1). Each point is the mean ± SEM of six to 17 replicates (SEM was, on average, <11% of the mean). Cytosolic exchange half-times are listed in parentheses (SEM < 15% of the mean).
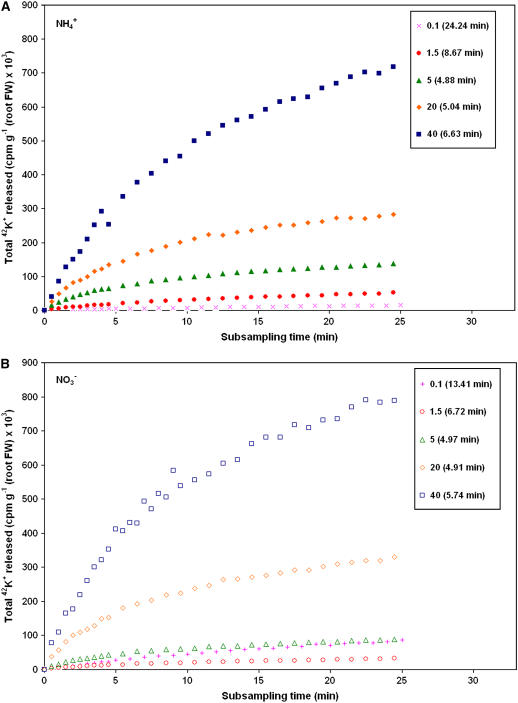
Figure 2.
Comparison of 42K+ accrual in growth solution surrounding roots of barley seedlings grown under five K+ concentrations and two N treatments, NH4+ (A) and NO3− (B). Plots have been corrected for differences in root mass and tracer activity (to the arbitrary value of 2 × 105 cpm μmol−1). Each point is the mean ± SEM of three to 12 replicates (SEM was, on average, <10% of the mean). Cytosolic exchange half-times are listed in parentheses (SEM was <15% of the mean).
Similar trends were found in subsampling experiments, as seen in Figure 2, which shows 42K+ released from plant roots and accruing in the initially nonradioactive external medium (for details of this method, see Britto et al., 2006). This graph is qualitatively different from that in Figure 1, as it shows tracer released progressively over time, rather than a rate per se of tracer release. Nevertheless, the greater tendency for K+ to be lost from the plant under higher [K+]ext is clearly visible here, under both N conditions. The strong difference between cytosolic K+ exchange half-times under HATS and LATS conditions is also indicated in Figure 2, with more rapid exchange again prevailing at higher K+ supply.
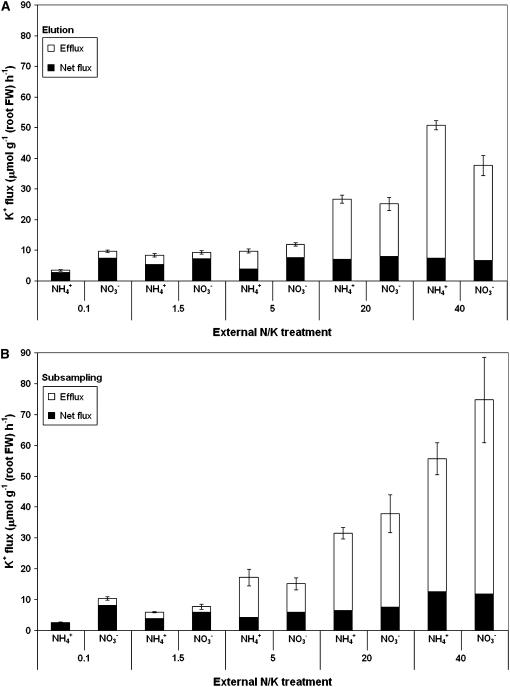
Figure 3 illustrates the dependence of influx and efflux, and the relative independence of the net flux term, on [K+]ext, as determined by elution and subsampling methods, respectively. With both methods, net K+ flux was clearly depressed under NH4+ nutrition at lower [K+]ext, but otherwise φnet was fairly uniform. By contrast, efflux, influx, and the ratio of the two dramatically increased with greater K+ provision. Efflux to influx ratios exceeded 70% at 20 mm [K+]ext and reached maximal values of 0.85 at the highest [K+]ext of 40 mm.
Figure 3.
Comparison of K+ component fluxes, as determined by elution (A) and subsampling (B), at five external K+ concentrations (as indicated, in mm) with two N sources (10 mm NH4+ or NO3−). Bars are divided into net flux (black segments) and efflux (white segments), which together comprise the influx term. Error bars refer to ±SEM of three to 17 replicates.
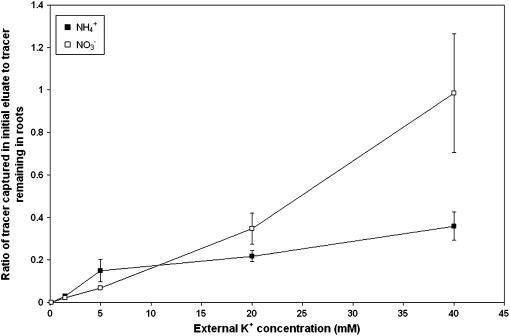
While fluxes measured by the two methods showed good agreement overall, subsampling yielded higher influx and efflux values at the two highest K+ conditions (20 and 40 mm), when plants were supplied with nitrate. With the subsampling protocol, we also observed an initial, nonexponential burst of tracer efflux that depended on [K+]ext, N source, and physical handling of the plant (see Britto et al., 2006). Figure 4 illustrates this dependence, in the form of a change in the ratio of this efflux burst, captured in the first eluate of the subsampling protocol, to the total root retention of tracer at the end of an experimental run. At the higher [K+]ext values (and particularly at 40 mm), this tracer burst was greater with nitrate-grown, relative to ammonium-grown, plants. This indicates greater membrane permeability of K+ in NO3−-grown plants at high [K+]ext, which cannot be accounted for in the elution protocol (Britto et al., 2006), and may therefore explain the discrepancy between methods under these specific conditions.
Figure 4.
Ratio of 42K+ captured in the initial eluate during a subsampling protocol to tracer remaining in roots after elution. Values are corrected for root mass and tracer activity. Black squares refer to seedlings grown with NH4+; white squares refer to NO3−. Each point is the mean ± SEM of three to 16 replicates.
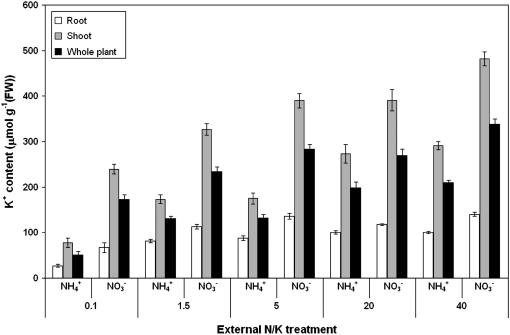
Figure 5 depicts the tissue K+ content of root, shoot, and total plant for each of the 10 treatments. These results agree with the trend shown for φnet values (Fig. 3), in that K+ accumulation is suppressed in low-K+, ammonium-grown plants, and K+ accumulation is relatively insensitive to [K+]ext. However, calculations based on a 10% daily growth rate of seedlings (Britto et al., 2001) and 16 h (equivalent to the light period) of sustained uptake at reported net fluxes (Fig. 3) reveal that these fluxes are, under all conditions, in excess of those required to achieve the tissue [K+] levels reported in Figure 5. This might, in part, be attributable to net K+ uptake not being maximally sustained throughout the day; indeed, φnet may even assume negative values under some conditions (Macduff and Dhanoa, 1996). The discrepancy between the reported values of φnet and tissue K+ concentrations may also be due to the fact that φnet determined by short-term tracer accumulation represents a “quasi-steady influx to the vacuole” (p. 1013) that exceeds the K+ accumulation rate by the amount of efflux from the vacuole (Cram, 1969).
Figure 5.
K+ tissue content of seedlings grown at five external K+ concentrations (as indicated, in mm) and two N sources (10 mm NH4+ or NO3−). Root content is represented by white bars, shoot content by gray bars, and whole-plant content by black bars. Error bars refer to ±SEM of six to 12 replicates. Root contents are expressed per gram root, shoot contents are expressed per gram shoot, and whole-plant contents are expressed per gram total tissue.
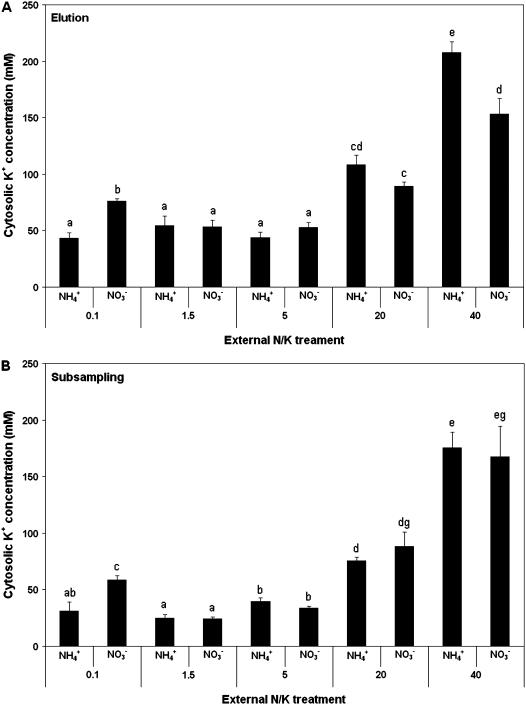
Cytosolic K+ concentrations, as determined using the two methods of 42K+-flux analysis, are shown in Figure 6 for the 10 growth conditions. With both methods, a distinct pattern of decreasing [K+]cyt was observed for plants grown with nitrate and with [K+]ext between the HATS condition of 0.1 mm and the lower end of the LATS scale (1.5 and 5 mm). This trend, however, was reversed with further increases of [K+]ext (to 20 and 40 mm), with the [K+]cyt values rising to 200 mm, dramatically above the HATS baseline. With ammonium feeding, suppression of [K+]cyt (relative to the nitrate treatment) was observed in the HATS range, and this suppression was maintained in the low end of the LATS range (up to 5 mm [K+]ext). Between 5 and 40 mm [K+]ext, however, a pattern of rising [K+]cyt was seen in NH4+-grown plants, in parallel with NO3−-grown plants.
Figure 6.
K+ concentration of root cytosolic compartment ([K+]cyt) for barley seedlings grown at five external K+ concentrations (as indicated, in mm) and two N sources (10 mm NH4+ or NO3−), determined by either elution (A) or subsampling (B) protocols. Error bars refer to ±SEM of six to 17 replicates (A) and ±SEM of three to six replicates (B). Different letters refer to significantly different means (P < 0.05, tested by ANOVA).
Table I shows electrophysiological measurements of plasma membrane electrical potentials (ΔΨ), confirming the progressive depolarization of ΔΨ as [K+]ext increases, and we found generally lower ΔΨ values in the presence of NH4+. Table I combines compartmentation, flux, and electrophysiological data with Nernst and Ussing-Teorell analysis to determine the direction of active K+ flux across the plasma membrane. The analysis shows that, while influx is the active step in the HATS range, efflux is the energy-requiring step under all LATS conditions.
Table I.
Directly measured plasma membrane electrical potentials (ΔΨ) and direction of energy requiring flux, as determined by the Nernst and Ussing-Teorell equation
External concentrations of NH4+ and NO3− were 10 mm. Membrane potentials are the means ± sd of seven to 38 replicates. Cytosolic activity estimates used in the calculations were found using both elution and subsampling protocols; the direction of the energy-requiring flux was the same regardless of protocol.
| N Treatment | [K+]ext | ΔΨ | Energy-Requiring Flux |
|---|---|---|---|
| mm | mV | ||
| NH4+ | 0.1 | −118 ± 10 | Influx |
| 1.5 | −123 ± 18 | Efflux | |
| 5 | −119 ± 9 | Efflux | |
| 20 | −59 ± 6 | Efflux | |
| 40 | −51 ± 9 | Efflux | |
| NO3− | 0.1 | −141 ± 11 | Influx |
| 1.5 | −125 ± 12 | Efflux | |
| 5 | −119 ± 13 | Efflux | |
| 20 | −86 ± 17 | Efflux | |
| 40 | −86 ± 10 | Efflux |
DISCUSSION
Is Cytosolic Potassium Homeostatically Controlled in the LATS?
It has become accepted that the cytosolic K+ pool of plant cells is maintained homeostatically, with high stringency, at approximately 100 mm (Walker et al., 1996, 1998; Leigh, 2001). This is believed to be necessary for the maintenance of critical enzymatic and osmotic functions, even though studies have shown that concentrations far below 100 mm can be sufficient for maximal enzyme activation of K+-dependent enzymes (Nitsos and Evans, 1969; Memon et al., 1985b) and the osmotic functions of K+ may be assumed by compatible solutes or other ions (Harvey et al., 1981). The strict maintenance of the cytosolic potassium pool ([K+]cyt) is thought to be achieved by K+ fluxes from the vacuole and the external medium. However, prolonged K+ starvation or exposure to toxicants such as Na+ and NH4+ have been shown to suppress [K+]cyt (Jeschke and Stelter, 1976; Harvey et al., 1981; Mills et al., 1985; Hajibagheri et al., 1987, 1988, 1989; Walker et al., 1996, 1998; Flowers and Hajibagheri, 2001; Carden et al., 2003; Kronzucker et al., 2003b). Furthermore, closer examination of published studies shows a range of [K+]cyt values, in the absence of toxicants, between 43 and 320 mm (Pierce and Higinbotham, 1970; Leigh and Wyn Jones, 1984; Memon et al., 1985a). In addition, in prior work, we demonstrated the very surprising finding that raising the external K+ supply from two HATS conditions of 0.02 and 0.1 mm (under which [K+]cyt constancy was observed) to a HATS-LATS transitional condition of 1.5 mm (Kochian and Lucas, 1982; Kochian et al., 1985) also brought about a lowering of [K+]cyt in root cells of barley (Kronzucker et al., 2003b). In this study, we have undertaken a comprehensive analysis of fluxes and subcellular compartmentation of K+ in the LATS range of transport, largely to investigate whether this dynamic condition is a general characteristic of the LATS.
Figure 6, in agreement with our previous work (Kronzucker et al., 2003b), shows that, in nitrate-grown plants, the K+-sufficient, HATS-LATS transitional condition of 1.5 mm presents lowered average cytosolic K+ concentrations, compared to the (also K+-sufficient) HATS condition, dropping by more than 50% when examined via subsampling (Fig. 6B). [K+]ext-dependent [K+]cyt suppression occurred despite the substantial increases in total tissue [K+] in both roots and shoots at 1.5 mm (Fig. 5), and runs contrary to the paradigm of [K+]cyt constancy under K+ sufficiency. Higher tissue K+ at 1.5 mm [K+]ext indicates that the vacuolar K+ pool increased even as [K+]cyt declined. K+ supply to the cytosol, either from the vacuole or the external medium, therefore should not be limiting under these conditions. [K+]ext-dependent [K+]cyt suppression also occurred despite the nearly identical net flux and influx of K+ under the 0.1 mm and 1.5 mm [K+]ext conditions (in nitrate-grown plants). In addition, [K+]cyt was found to vary substantially between 5 and 40 mm [K+]ext, despite fairly similar net fluxes under these conditions. These discrepancies, like those between apparent net fluxes and K+ tissue content, reflect differential correspondence, among K+ treatments, between the apparent net flux (as measured by short-term radiotracer accumulation) and the “quasi-steady flux to the vacuole” (Cram, 1969; p. 1013).
In this part of the applied range of [K+]ext (0.1–1.5 mm), however, [K+]ext-dependent [K+]cyt suppression was not observed with NH4+ as N source, but this was because [K+]cyt was already suppressed in the HATS condition by NH4+, an ion that has well-known inhibitory effects upon high-affinity K+ transport (Vale et al., 1987, 1988; Hirsch et al., 1998; Spalding et al., 1999; Santa-Maria et al., 2000). Interestingly, even though [K+]cyt remained low in plants grown at 1.5 mm (and 5 mm) [K+]ext, with or without NH4+, no impairment of growth or visual symptoms of toxicity were observed; on the contrary, plants grown at 1.5 and 5 mm K+ had greater fresh weight in both roots and shoots than those grown at 0.1 mm (data not shown; see Britto and Kronzucker, 2002). This indicates that the maintenance of a strict [K+]cyt value is not essential.
While little change in [K+]cyt occurred between 1.5 and 5 mm [K+]ext, the dynamic nature of [K+]cyt again became evident as [K+]ext was increased beyond 5 mm (Fig. 6). At these high external concentrations, a recovery of [K+]cyt was observed, with [K+]cyt values at 20 mm already exceeding those of the HATS condition and values at 40 mm approximately doubling those at 20 mm. Our results in barley are further supported by experiments conducted in rice (Oryza sativa L. cv IR-72), where [K+]cyt was found to decline from approximately 160 mm at 0.1 mm [K+]ext to a minimum of approximately 40 mm at 1.5 mm [K+]ext, followed by a dramatic rise to approximately 250 mm at 40 mm [K+]ext (M.W. Szczerba, D.T. Britto, and H.J. Kronzucker, unpublished data).
The variability in LATS-range cytosolic K+ pools demonstrated here stands in sharp contrast to the relative constancy in the HATS range (Kronzucker et al., 2003b), and reveals a distinguishing characteristic between the two transport modes, which must be related to differences in unidirectional and net flux functions. Furthermore, it has inescapable consequences for the energetics of plasma membrane transport (see below). The variability in [K+]cyt values we have observed also stands in contrast to the constancy of cellular K+ activity (around 75 mm) that has been observed, also in barley roots, by use of potassium-selective microelectrodes under a variety of K+-replete conditions (Walker et al., 1996). Although the values obtained by this method and ours are quite similar overall, Walker et al. (1996) failed to observe variability in this pool except under K+ deprivations, for reasons that are not resolved. However, the reliability of microelectrode measurements is called into question by a comparison of two studies undertaken by the same group in the same plant system; in one study, a pronounced decline in cytosolic K+ activity (aK) with prolonged exposure to K+ deprivation (2 μm) was observed (Walker et al., 1998), while, in the other study, “growing the roots in CaSO4 [with 2 μm K+] did not significantly affect the way…cytosolic aK behaved” (Walker et al., 1996; p. 10511).
Studies using other methods have shown that [K+]cyt is not necessarily constant but is suppressed by sodium provision. For instance, a study with x-ray microanalysis showed that [K+]cyt drops by nearly 50% in two cultivars of barley when exposed to high NaCl concentrations (Flowers and Hajibagheri, 2001; also see Hajibagheri et al., 1987, 1989). Similar Na+-dependent declines in [K+]cyt have also been seen using longitudinal ion profiling (Jeschke and Stelter, 1976) and organelle fractionation (Robinson et al., 1983). These two methods (x-ray microanalysis and longitudinal ion profiling) have also been shown to give results consistent with those obtained using compartmental analysis by tracer efflux (Hajibagheri et al., 1988).
Futile K+ Cycling across the Plasma Membrane in the LATS
Analysis of K+ exchange between the external medium and cytosol was an equally important aspect of this study because of the role that K+ influx and efflux play in the regulation of [K+]cyt. Our previous work had suggested that an increase in K+ supply brings about increases both in the ratio of efflux to influx and in the degree of cytosolic K+ cycling. Aspects of this cellular behavior have been observed for other ions, such as Na+ (Cheeseman, 1982; Essah et al., 2003; Davenport et al., 2005), NH4+ (Min et al., 1999; Britto et al., 2001, 2002; Kronzucker et al., 2003a), NO3− (Kronzucker et al., 1999; Min et al., 1999; Scheurwater et al., 1999), and Cl− (Britto et al., 2004), but have, until now, not been systematically demonstrated for potassium. Figures 1 and 2 show that, regardless of experimental or analytical procedure, plants grown under the HATS condition of 0.1 mm display a much longer half-time of cytosolic K+ exchange (with values very similar to those reported by Jeschke [1982], Kochian and Lucas [1982], Memon et al. [1985a], and Kronzucker et al. [2003b]) relative to the four LATS conditions examined, with plants grown at the intermediate [K+]ext of 1.5 mm showing a transitional half-time. This pattern was unaffected by N source and demonstrates that the turnover of the cytosolic K+ pool is accelerated in the LATS condition.
At 5 mm [K+]ext and above, the acceleration of cytosolic K+ cycling takes on a striking characteristic: the extent of K+ efflux relative to influx (and to net flux) begins to increase, becoming most dramatic at 20 and 40 mm [K+]ext, regardless of N source. At 40 mm, the ratio of efflux to influx exceeds 80%, indicating the increasing intensity of futile K+ cycling at the plasma membrane, reminiscent of the futile cycling previously reported for NH4+ in several plant systems (Min et al., 1999; Britto et al., 2001, 2002; Kronzucker et al., 2003b). Importantly, for the energetics of transport (see below), this change in the partitioning of influx toward efflux and net flux does not come about merely as a redistribution of a constant influx but occurs in the context of a greatly increased (channel-mediated) influx. In the most dramatic case, K+ influx into NH4+-fed plants increases by more than 20-fold over the 0.1 to 40 mm [K+]ext range. This is indicative of a switch between the low end of the [K+]ext range, in which NH4+-sensitive HATS transporters preside, to the LATS section of the range, where NH4+-insensitive K+ channels and nonselective cation channels are the dominant means of K+ influx (Hirsch et al., 1998; Santa-Maria et al., 2000; Véry and Sentenac, 2002) and can sustain K+ acquisition even when external [NH4+] is high, alleviating the toxicity of the NH4+ ion (Britto and Kronzucker, 2002). By contrast, with influx and efflux trends, there is little increase in the net K+ flux across the conditions examined, as paralleled by the very moderate increases in tissue K+ across the same conditions (Fig. 5; see Asher and Ozanne, 1966). Thus, the degree of luxury K+ uptake engaged in by the roots becomes very pronounced. A consequence of very high ratios of efflux to influx is that unidirectional K+ influx, when measured by protocols that do not quantify efflux, becomes increasingly difficult to measure as [K+]ext rises (Cram, 1969; Britto and Kronzucker, 2001; see also Essah et al., 2003; Davenport et al., 2005). This problem is exacerbated as K+ exchange half-times decrease in the LATS range (Britto and Kronzucker, 2001). Even in our present measurements, which use efflux detection by radiotracing, there are some discrepancies between the two methods used, when [K+]ext becomes very large (20–40 mm), and when NH4+ is the N source.
Energetics of the LATS Flux Condition
Across the 10 conditions studied here, the [K+]ext-dependent variations in electrical polarization of the plasma membrane (Table I), with increasing depolarization at higher [K+]ext, are in broad agreement with published values (Etherton and Higinbotham, 1960; Higinbotham et al., 1964; Pitman and Saddler, 1966; Cheeseman and Hanson, 1979; Beilby and Blatt, 1986; Newman et al., 1987; Kochian et al., 1989). This trend, in conjunction with our reported cytosolic K+ pool-size dynamics (Fig. 6) and highly variable K+ fluxes into and out of the cytosol (Fig. 3), results in a wide range of conditions governing the energetics of plasma membrane K+ transport. However, as shown in Table I, all experimental treatments except for the HATS condition of 0.1 mm [K+]ext reveal that, of the two dominant fluxes (unidirectional influx and efflux), efflux is the active, energy-requiring transport step. This scenario is analogous to that prevailing in plants exposed to elevated concentrations of NH4+ or Na+, where passive, channel-mediated influx (“leak”) is accompanied by active efflux (“pump”) of the ions (Cheeseman 1982; Britto et al., 2001; Kronzucker et al., 2001; Essah et al., 2003; Davenport et al., 2005). In the case of Na+, this extrusion mechanism is thought to occur predominantly via the Na+/H+ antiporter known as SOS1 (Apse and Blumwald, 2002). For potassium, the operation of K+/H+ antiporters has also been demonstrated (Hassidim et al., 1990; Cooper et al., 1991), although a molecular identification of these transporters in the plasma membrane is as yet outstanding (Pardo et al., 2006). The identity and contribution of such active transporters to K+ efflux are as important to the low-affinity transport condition as are the primary means of K+ influx and therefore need to be investigated with rigor.
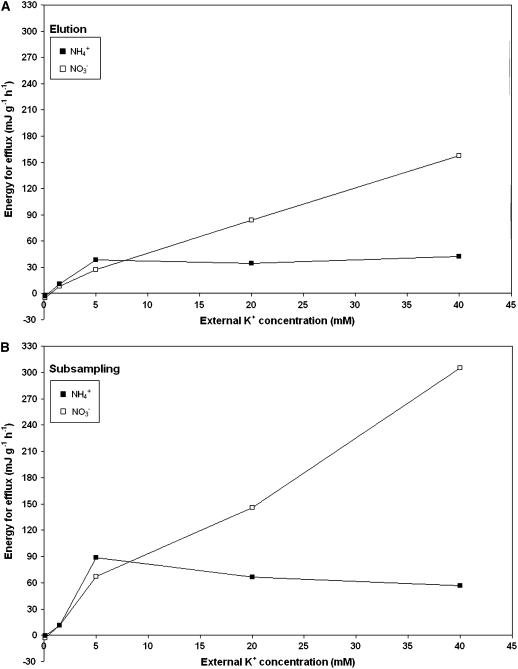
The specific energy requirements for K+ efflux under each condition in our study are shown in Figure 7. While elution and subsampling methods yield different absolute energy values (due to differences in flux and pool-size estimates between methods), the overall pattern is very similar: efflux under all LATS-range conditions requires energy, with the energy demand for K+ efflux in nitrate-grown plants substantially exceeding that in ammonium-grown plants when [K+]ext ≥ 20 mm. The more depolarized plasma membrane in NH4+-grown plants underlies the lesser energy requirement for LATS-range K+ efflux in the presence of NH4+.
Figure 7.
Energy necessary to drive K+ efflux from barley root cells, as determined by either elution (A) or subsampling (B) protocols. Negative values correspond to passive efflux, while positive values correspond to an active efflux. Black squares refer to NH4+-grown plants, and white squares refer to NO3−-grown plants.
We previously demonstrated that barley plants suffering from NH4+ toxicity actively extrude NH4+ from the cytosolic compartment of root cells, and we partially attributed the toxic effect to this energy demand (Britto et al., 2001; Kronzucker et al., 2001). Calculation shows that, at higher [K+]ext in the presence of NO3−, the energy requirement for K+ efflux is in fact greater than that for NH4+. However, plants in this study show no visible symptoms of toxicity or growth impairment; hence, this additional energy burden does not appear to have ill consequences. This is evidently because, unlike plants grown under elevated NH4+ concentrations and low [K+]ext, plants grown with abundant K+ (and with either NH4+ or NO3− as N source) are photosynthetically competent and indeed display increased rates of photosynthesis and carbon flow to the roots with increases [K+]ext (Hartt, 1970; Viro and Haeder, 1971; Peoples and Koch, 1979; Cakmak, 2005). Thus, the energy-intensive processes of active K+ efflux demonstrated here may have detrimental consequences for plant survival under energy-limiting conditions such as shade growth, given that roots can expend 50% to 70% of their cellular energy stores toward ion transport (Poorter et al., 1991; Scheurwater et al., 1999). By contrast, under agriculturally common high-irradiance conditions, where there may be an imbalance between the energy absorbed by photosystems and that utilized by metabolism (Hüner et al., 1998), the energy dissipated in futile ion cycling at the plasma membrane may in fact confer a fitness advantage in the field.
MATERIALS AND METHODS
Plant Culture
Seeds of barley (Hordeum vulgare L. cv. Klondike) were surface sterilized for 10 min in 1% sodium hypochlorite and germinated under acid-washed sand for 3 d prior to placement in 4-L vessels containing aerated hydroponic growth medium containing modified one-quarter-strength Johnson's solution, at pH 6 to 6.5, for an additional 4 d. The solution was modified to provide five concentrations of potassium (as K2SO4), at 0.1, 1.5, 5, 20, and 40 mm, and either NO3− [as Ca(NO3)2] or NH4+ [as (NH4)2SO4], at 10 mm. Solutions were exchanged frequently to ensure plants were at a nutritional steady state. Plants were cultured in walk-in growth chambers under fluorescent lights (Philips Econ-o-W, F96T12), with an irradiation of 200 μmol photons m−2 s−1 at plant height, for 16 h d−1. Daytime temperature was 20°C, nighttime temperature was 15°C, and relative humidity was approximately 70%.
Flux Experiments
An elution method and a subsampling method (referred to in previous work as compartmental analysis by tracer efflux and integrated flux analysis, respectively; see Britto et al., 2006) were used to estimate subcellular fluxes and compartmental pool sizes (for details, see Lee and Clarkson 1986; Siddiqi et al., 1991; Kronzucker et al., 1995, 2003b; Britto et al., 2001, 2006). With both methods, each replicate consisted of five plants held together at the shoot base by a plastic collar. Roots of these plants were labeled for 60 min in growth solution containing the radiotracer 42K (t½ = 12.36 h) and then desorbed for 30 min to measure efflux, either by a complete, periodic exchange of the nonradioactive solution bathing the roots (elution method) or by removing aliquots of the external medium as it became progressively labeled (subsampling method; for more details, see below and Britto et al., 2006). Labeling and desorption solutions were identical to growth solutions. 42K+ was provided by the McMaster University Nuclear Reactor (Hamilton, Ontario, Canada). Solutions were mixed using a fine stream of air bubbles. All radioactive samples were analyzed using a γ-counter, corrected for isotopic decay during experimentation (model 5003, Quantum Cobra Series II; Canberra-Packard).
In elution experiments, labeled seedlings were attached to efflux funnels and washed with successive 13-mL aliquots of desorption solution, identical to the growth solution. Elution protocol was timed as follows: 15 s (four times), 20 s (three times), 30 s (twice), 40 s (once), 50 s (once), 1 min (five times), 1.25 min (once), 1.5 min (once), 1.75 min (once), and 2 min (eight times). Immediately following elution, shoots were detached from roots and roots spin-dried in a low-speed centrifuge for 30 s prior to weighing. 42K+ from eluates, roots, and shoots, and centrifugates were γ-counted. Linear regression of the function log (42K+ efflux) versus time (or, as expressed in natural logarithms, ln φco(t)* = ln φco(i)* − kt, where φco(t)* is 42K+ efflux at elution time t, φco(i)* is initial 42K+ efflux, and k is the rate constant of the exponential decline in 42K+ efflux; exchange half-times were found from the equation t½ = 0.693/k) was used to resolve the kinetics of the slowest-exchanging phase in these experiments, which represents tracer exchange with the cytosolic compartment (Macklon, 1975; Kochian and Lucas, 1982; Memon et al., 1985a; Kronzucker et al., 2003b). The slope k of the regression line thus represents the kinetic exchange constant for the cytosol, while the intercept represents initial tracer efflux (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995). Chemical efflux, φco, was determined from this intercept, divided by the specific activity of the cytosol at the end of the 60-min labeling period (see Kronzucker et al., 2003b). Net flux, φnet, was found using total-plant 42K+ retention after desorption (for details, see Kronzucker et al., 2003b). Influx, φoc, was calculated from the sum of φnet and φco. [K+]cyt was determined using the flux-turnover equation [K+]cyt = Ω φoc/k, where Ω is a proportionality constant correcting for the cytosolic volume being approximately 5% of total tissue (Britto and Kronzucker, 2001; Kronzucker et al., 2003b).
In subsampling experiments, plant bundles were labeled and desorbed in 30 mL of nutrient solution, except for the 0.1 mm K+ treatment, in which a 100-mL solution was used, to minimize K+ depletion. After 1 h of labeling, seedlings were immersed for 5 s in nonradioactive solution to remove superficial tracer, then transferred sequentially to two desorption vessels for 5 and 25 min, respectively. Three-milliliter aliquots were removed (and replaced with fresh nonradioactive solution every 30 s) from the first desorption vessel and every 30 s from the second desorption vessel until the 5-min time point, after which samples were taken every minute. Tracer collected over time was quantified as described by Britto et al. (2006). Plant tissues were processed and γ-counted as described above for elution experiments. 42K+ released over time was resolved using exponential equations of the form At = A0 (1 − e−kt), determined by nonlinear, least-squares regression (Britto et al., 2006). In this equation, At represents cumulative tracer released at time t, and A0 represents maximal tracer released over the entire time course. As with the elution method, the kinetic constant (k) for cytosolic exchange was found directly from exponential regressions. Efflux was calculated by differentiating the exponential equation above with respect to time (dAt/dt = k × Ao × e−kt = φco* at t = 0) and dividing by the specific activity of the cytosol (Britto et al., 2006). Cytosolic half-time and [K+]cyt, φnet, and φoc were calculated as stated above for elution experiments.
Tissue K+ Content
To measure tissue K+ content, roots of barley seedlings were desorbed for 5 min in 10 mm CaSO4 to remove extracellular K+. Roots and shoots were then separated and weighed. Tissue was oven dried for a minimum of 72 h at 80°C to 85°C, reweighed, pulverized, and digested with 30% HNO3 for a minimum of 72 h. K+ concentration was determined using a single-channel flame photometer (Digital Flame Analyzer model 2655-00; Cole-Parmer).
Electrophysiology
Membrane potential differences were measured as described elsewhere (Britto et al., 2001). In short, single roots of intact barley seedlings were secured in a clear plastic cuvette filled with nutrient solution and mounted on the stage of an inverted binocular compound light microscope (Alphaphot; Nikon). Impalements were typically performed at 2 to 6 cm from the root tip, using a Huxley-style micromanipulator (MX310R; SD Instruments), and membrane potentials were measured using a DUO 773 electrometer (World Precision Instruments). Microelectrodes were made from single-barreled borosilicate glass micropipettes filled with 3 m KCl and had tip diameters less than 1 μm (confirmed using scanning electron microscopy). Reference electrodes consisted of borosilicate micropipettes filled with 2% (w/v) agar. Impalements were considered successful only when a steady electrical potential difference was maintained for at least 2 min. Activity coefficients (γ) for the various growth-medium concentrations of K+ were estimated using the Debye-Hückel-Onsager equation, adapted for the monovalent cation K+:
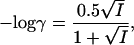 |
where I is ionic strength of the medium (Jander and Blasius, 1988). The cytosolic K+ activity coefficient used was 0.77 (Macklon, 1975). The Nernst potential (EN) for K+ was determined using the following equation:
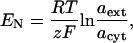 |
where R is the universal gas constant, T was ambient temperature (293.15 K), z is the ionic charge of the species (+1 for K+), F is the Faraday constant, and aext and acyt are the external and cytosolic activities for K+, respectively. To determine whether transport of K+ was active or passive, the Ussing-Teorell equation was used:
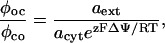 |
with φoc and φco representing influx and efflux, respectively, from the cytosolic compartment, ΔΨ the measured membrane potential, and a, z, F, R, and T as described above.
Acknowledgments
We thank M. Butler at McMaster University in Hamilton, Ontario, Canada, for supplying the 42K+ required to conduct these experiments. We also thank R.R. Lew and A.C. Mason for technical assistance, and M. Sheriff, P.S. Tehrani, S. MacKay, N. Alingary, and A.B. Vesterberg for assistance with experiments.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Herbert J. Kronzucker (herbertk@utsc.utoronto.ca).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.082701.
References
- Adams F (1971) Soil solution. In EW Carson, ed, The Plant Root and Its Environment. University Press of Virginia, Charlottesville, VA, pp 441–481
- Apse MP, Blumwald E (2002) Engineering salt tolerance in plants. Curr Opin Biotechnol 13: 146–150 [DOI] [PubMed] [Google Scholar]
- Asher CJ, Ozanne PG (1966) Growth and potassium content of plants in solution cultures maintained at constant potassium concentrations. Soil Sci 103: 155–161 [Google Scholar]
- Ashley MK, Grant M, Grabov A (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57: 425–436 [DOI] [PubMed] [Google Scholar]
- Beilby MJ, Blatt MR (1986) Simultaneous measurements of cytoplasmic K+ concentration and the plasma membrane electrical parameters in single membrane samples of Chara corallina. Plant Physiol 82: 417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ (2001) Can unidirectional influx be measured in higher plants? A mathematical approach using parameters from efflux analysis. New Phytol 150: 37–47 [Google Scholar]
- Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159: 567–584 [Google Scholar]
- Britto DT, Ruth TJ, Lapi S, Kronzucker HJ (2004) Cellular and whole-plant chloride dynamics in barley: insights into chloride-nitrogen interactions and salinity responses. Planta 218: 615–622 [DOI] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ (2001) Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98: 4255–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ (2002) Subcellular NH4+ flux analysis in leaf segments of wheat (Triticum aestivum). New Phytol 155: 373–380 [DOI] [PubMed] [Google Scholar]
- Britto DT, Szczerba MW, Kronzucker HJ (2006) A new, non-perturbing, sampling procedure in tracer exchange measurements. J Exp Bot 57: 1309–1314 [DOI] [PubMed] [Google Scholar]
- Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168: 521–530 [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol 131: 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM (1982) Pump-leak sodium fluxes in low salt corn roots. J Membr Biol 70: 157–164 [Google Scholar]
- Cheeseman JM, Hanson JB (1979) Energy-linked potassium influx as related to cell potential in corn roots. Plant Physiol 64: 842–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Lerner HR, Reinhold L (1991) Evidence for a highly specific K+/H+ antiporter in membrane vesicles from oil-seed rape hypocotyls. Plant Physiol 97: 1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram WJ (1969) Short term influx as a measure of influx across the plasmalemma. Plant Physiol 44: 1013–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Ehrhardt T, Müller-Röber B (1997) New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J 16: 2565–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiol 137: 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E, Rains DW, Elzam OE (1963) Resolution of dual mechanisms of potassium absorption by barley roots. Proc Natl Acad Sci USA 49: 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B, Higinbotham N (1960) Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science 131: 409–410 [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Hajibagheri MA (2001) Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 231: 1–9 [Google Scholar]
- Hajibagheri MA, Flowers TJ, Collins JC, Yeo AR (1988) A comparison of the methods of X-ray-microanalysis, compartmental analysis and longitudinal ion profiles to estimate cytoplasmic ion concentrations in two maize varieties. J Exp Bot 39: 279–290 [Google Scholar]
- Hajibagheri MA, Harvey DMR, Flowers TJ (1987) Quantitative ion distribution within root-cells of salt-sensitive and salt-tolerant maize varieties. New Phytol 105: 367–379 [DOI] [PubMed] [Google Scholar]
- Hajibagheri MA, Yeo AR, Flowers TJ, Collins JC (1989) Salinity resistance in Zea mays: fluxes of potassium, sodium and chloride, cytoplasmic concentrations and microsomal membrane lipids. Plant Cell Environ 12: 753–757 [Google Scholar]
- Hartt CE (1970) Effect of potassium deficiency upon translocation of 14C in detached blades of sugarcane. Plant Physiol 45: 183–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DMR, Hall JL, Flowers TJ, Kent B (1981) Quantitative ion localization within Suaeda maritima leaf mesophyll cells. Planta 151: 555–560 [DOI] [PubMed] [Google Scholar]
- Hassidim M, Braun Y, Lerner HR, Reinhold L (1990) Na+/H+ and K+/H+ antiport in root membrane vesicles isolated from the halophyte Atriplex and the glycophyte cotton. Plant Physiol 94: 1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N, Etherton B, Foster RJ (1964) Effect of external K, NH4, Na, Ca, Mg, and H ions on the cell transmembrane electropotential of Avena coleoptile. Plant Physiol 39: 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Hüner NPA, Oquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3: 224–230 [Google Scholar]
- Jander G, Blasius E (1988) Lehrbuch der analytischen und präparativen anorganischen Chemie, Ed 12. S. Hirzel Publishing House, Stuttgart, Germany, pp 54–55
- Jeschke WD (1970) Der Influx von Kaliumionen bei Blättern von Elodea densa, Abhängigkeit vom Licht, von der Kaliumkonzentration und von der Temperatur. Planta 91: 111–128 [DOI] [PubMed] [Google Scholar]
- Jeschke WD (1982) Shoot-dependent regulation of sodium and potassium fluxes in roots of whole barley seedlings. J Exp Bot 135: 601–618 [Google Scholar]
- Jeschke WD, Stelter W (1976) Measurement of longitudinal ion profiles in single roots of Hordeum and Atriplex by use of flameless atomic absorption spectroscopy. Planta 128: 1076–1080 [DOI] [PubMed] [Google Scholar]
- Kader MA, Lindberg S (2005) Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. J Exp Bot 56: 3149–3158 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ (1982) Potassium transport in corn roots. I. Resolution of kinetics into a saturable and linear component. Plant Physiol 70: 1723–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Shaff JE, Lucas WJ (1989) High-affinity K+ uptake in maize roots: a lack of coupling with H+ efflux. Plant Physiol 91: 1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Xin-zhi J, Lucas WJ (1985) Potassium transport in corn roots. IV. Characterization of the linear component. Plant Physiol 79: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT, Davenport RJ, Tester M (2001) Ammonium toxicity and the real cost of transport. Trends Plant Sci 6: 335–337 [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY (1999) Inhibition of nitrate uptake by ammonium in barley. Analysis of component fluxes. Plant Physiol 120: 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM (1995) Analysis of 13NH4+ efflux in spruce roots (a test case for phase identification in compartmental analysis). Plant Physiol 109: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM, Britto DT (2003. a) Root ammonium transport efficiency as a determinant in forest colonization patterns: an hypothesis. Physiol Plant 117: 164–170 [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT (2003. b) Cytosolic potassium homeostasis revisited: 42K-tracer analysis in Hordeum vulgare L. reveals set-point variations in K+. Planta 217: 540–546 [DOI] [PubMed] [Google Scholar]
- Lee RB, Clarkson DT (1986) 13N studies of nitrate fluxes in barley roots. 1. Compartmental analysis from measurements of 13N efflux. J Exp Bot 37: 1753–1767 [Google Scholar]
- Leigh RA (2001) Potassium homeostasis and membrane transport. J Plant Nutr Soil Sci 164: 193–198 [Google Scholar]
- Leigh RA, Wyn Jones RG (1984) A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol 97: 1–13 [Google Scholar]
- Maathuis FJM, Sanders D (1993) Energization of potassium uptake in Arabidopsis thaliana. Planta 191: 302–307 [Google Scholar]
- Maathuis FJM, Sanders D (1997) Regulation of K+ absorption in plant root cells by external K+: interplay of different plasma membrane K+ transporters. J Exp Bot 48: 451–458 [DOI] [PubMed] [Google Scholar]
- Macduff JH, Dhanoa MS (1996) Diurnal and ultradian rhythms in K+ uptake by Trifolium repens under natural light patterns: evidence for segmentation at different root temperatures. Physiol Plant 98: 298–308 [Google Scholar]
- Macklon AES (1975) Cortical cell fluxes and transport to the stele in excised root segments of Allium cepa L. I. Potassium, sodium and chloride. Planta 122: 109–130 [DOI] [PubMed] [Google Scholar]
- Memon AR, Saccomani M, Glass ADM (1985. a) Efficiency of potassium utilization by barley varieties: the role of subcellular compartmentation. J Exp Bot 36: 1860–1876 [Google Scholar]
- Memon AR, Siddiqi MY, Glass ADM (1985. b) Efficiency of K+ utilization by barley varieties: activation of pyruvate kinase. J Exp Bot 36: 79–90 [Google Scholar]
- Mills D, Robinson K, Hodges TK (1985) Sodium and potassium fluxes and compartmentation in roots of Atriplex and oat. Plant Physiol 78: 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ (1999) A comparative study of fluxes and compartmentation of nitrate and ammonium in early-successional tree species. Plant Cell Environ 22: 821–830 [Google Scholar]
- Morgan MA, Jackson WA (1984) Reciprocal ammonium transport into and out of plant roots: modifications by plant nitrogen status and elevated root ammonium concentration. J Exp Bot 40: 207–214 [Google Scholar]
- Newman IA, Kochian LV, Grusak MA, Lucas WJ (1987) Fluxes of H+ and K+ in corn roots: characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol 84: 1177–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsos RE, Evans HJ (1969) Effects of univalent cations on the activity of particulate starch synthetase. Plant Physiol 44: 1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57: 1181–1199 [DOI] [PubMed] [Google Scholar]
- Peoples TR, Koch DW (1979) Role of potassium in carbon dioxide assimilation in Medicago sativa L. Plant Physiol 63: 878–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson S, Kasimir-Klemedtsson Ǻ (1990) Influx and efflux of K+ in sunflower roots after transfer between solutions with different K+ concentrations. Physiol Plant 79: 686–692 [DOI] [PubMed] [Google Scholar]
- Pierce WS, Higinbotham N (1970) Compartments and fluxes of K+, Na+ and Cl− in Avena coleoptile cells. Plant Physiol 46: 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MG, Saddler HDW (1966) Active sodium and potassium transport in cells of barley roots. Proc Natl Acad Sci USA 57: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Vanderwerf A, Atkin OK, Lambers H (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiol Plant 83: 469–475 [Google Scholar]
- Reisenauer HM (1966) Mineral nutrients in soil solution. In PL Altman, DS Ditter, eds, Environmental Biology. Federation of American Societies for Experimental Biology, Bethesda, MD, pp 507–508
- Robinson SP, Downton WJS, Millhouse JA (1983) Photosynthesis and ion content of leaves and isolated-chloroplasts of salt-stressed spinach. Plant Physiol 73: 238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Maria GE, Danna CH, Czibener C (2000) High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiol 123: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HW, MacKown CT, Leggett JE (1984) Potassium ammonium uptake interactions in tobacco seedlings. J Exp Bot 35: 1060–1070 [Google Scholar]
- Scheurwater I, Clarkson DT, Purves JV, Van Rijt G, Saker LR, Welschen R, Lambers H (1999) Relatively large nitrate efflux can account for the high specific respiratory costs for nitrate transport in slow-growing grass species. Plant Soil 215: 123–134 [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ (1991) Studies of the uptake of nitrate in barley. III. Compartmentation of NO3−. J Exp Bot 42: 1455–1463 [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity. J Gen Physiol 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Skerrett IM (1999) Root ion channels and salinity. Sci Hortic (Amsterdam) 78: 175–235 [Google Scholar]
- Vale FR, Jackson WA, Volk RJ (1987) Potassium influx into maize root systems: influence of root potassium concentration and ambient ammonium. Plant Physiol 84: 1416–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale FR, Volk RJ, Jackson WA (1988) Simultaneous influx of ammonium and potassium into maize roots: kinetics and interactions. Planta 173: 424–431 [DOI] [PubMed] [Google Scholar]
- Véry AA, Sentenac H (2002) Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci 7: 168–175 [DOI] [PubMed] [Google Scholar]
- Véry AA, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Viro M, Haeder HE (1971) The effect of the potassium status of tomato plants on the transport of organic compounds to the fruits. In Proceedings of the 8th Colloquium of the International Potash Institute. International Potash Institute, Bern, pp 118–124
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A (2004) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27: 1–14 [Google Scholar]
- Walker DJ, Black CR, Miller AJ (1998) The role of cytosolic potassium and pH in the growth of barley roots. Plant Physiol 118: 957–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ (1996) Potassium homeostasis in vacuolated plant cells. Proc Natl Acad Sci USA 93: 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Sentenac H (1999) Plant ion channels: from molecular structures to physiological functions. Curr Opin Plant Biol 2: 477–482 [DOI] [PubMed] [Google Scholar]