Abstract
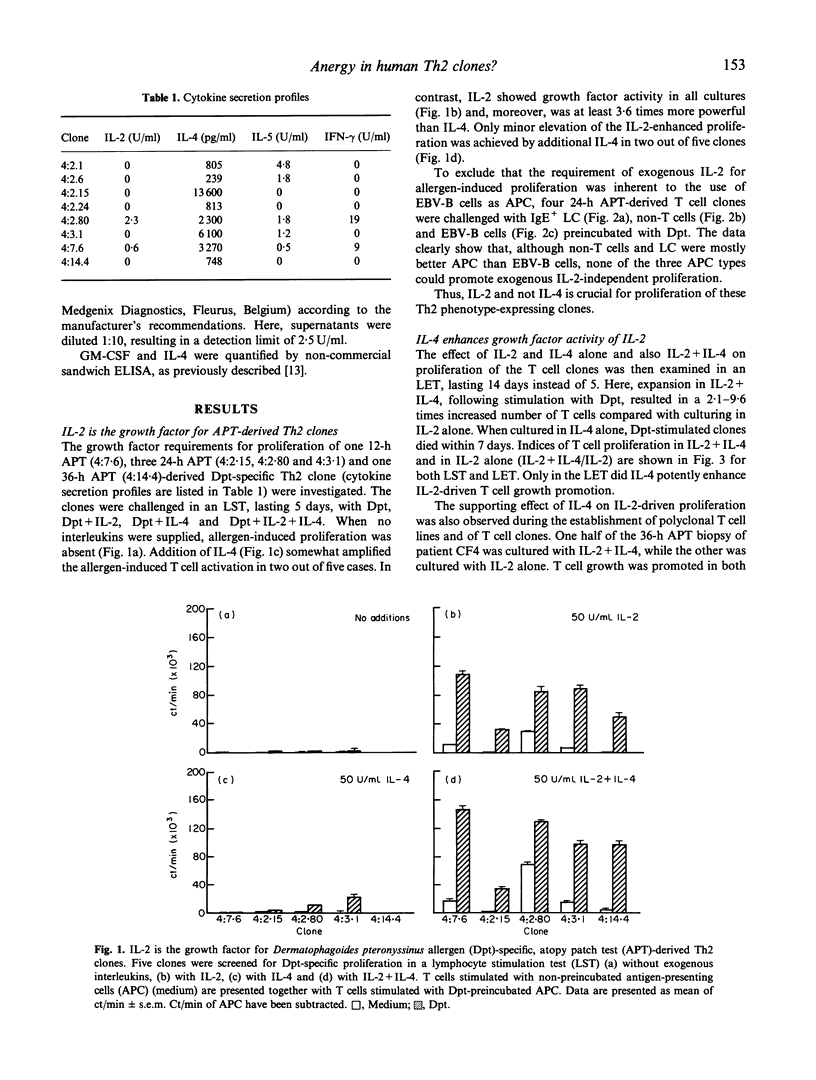
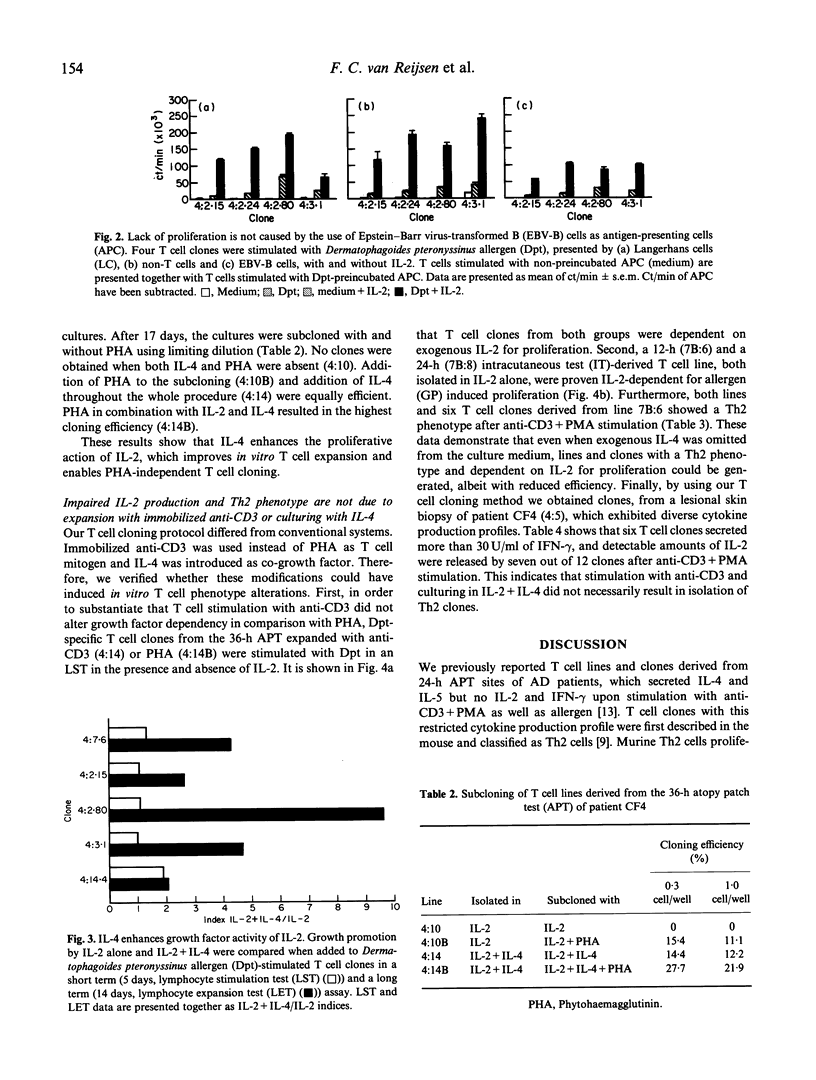
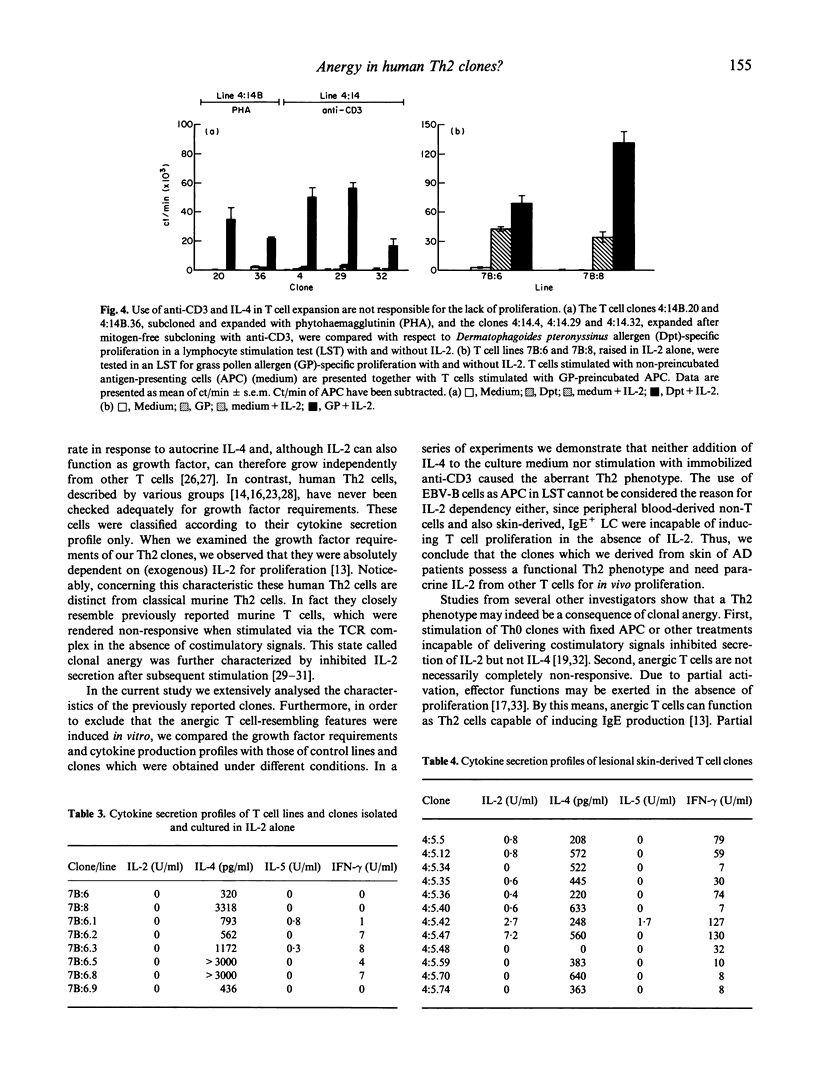
We previously reported the isolation of allergen-specific Th2 lines and clones from atopy patch test (APT) sites of atopic dermatitis (AD) patients. Upon stimulation with allergen or anti-CD3+ phorbol myristate acetate (PMA) IL-4 was released with or without IL-5, while no (or extremely low concentrations of) IL-2 and interferon-gamma (IFN-gamma) were detectable. A high IL-4/IFN-gamma ratio facilitates production of allergen-specific IgE, of which high levels are observed in AD patients. Here we show that the above mentioned Th2 cells are notably different from murine Th2 cells. Not IL-4, which is the autocrine acting growth factor for murine Th2 cells, but IL-2 was needed for proliferation of these human APT-derived Th2 lines and clones. Of significance, unless exogenous IL-2 was added, no proliferative response to allergen, presented by Epstein-Barr virus-transformed B (EBV-B) cells, non-T cells or IgE-bearing Langerhans cells (LC), occurred. Lack of proliferation and IL-2 production after full T cell receptor (TCR) triggering is a characteristic first described for in vitro anergized T cells. However, like the clones we describe in this study, anergic T cells may retain production of cytokines other than IL-2. A further resemblance between anergic T cells and the human Th2 clones reported here is that IL-4 can enhance IL-2-driven proliferation, but is not capable of inducing T cell growth by itself. The absence of IL-4-driven proliferation differentiates human Th2 cells from murine Th2 cells. Both produce IL-4 when stimulated in a cognate fashion, but only murine Th2 cells will proliferate. We conclude that the presently reported human Th2 cells are different from murine Th2 cells, in that they need other T cells to produce IL-2 required for their expansion. Moreover, the Th2 cells phenotypically resemble anergic T cells. As yet, however, we have no clue as to whether these features account for the current Th2 cells only or for human Th2 cells in general. We hypothesize that the Th2 phenotype of AD skin-derived, allergen-specific T cells may be induced in vivo by LC, which lack CD80, and therefore do not provide secondary signals through CD28-CD80 interaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruynzeel-Koomen C. A., Van Wichen D. F., Spry C. J., Venge P., Bruynzeel P. L. Active participation of eosinophils in patch test reactions to inhalant allergens in patients with atopic dermatitis. Br J Dermatol. 1988 Feb;118(2):229–238. doi: 10.1111/j.1365-2133.1988.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Abbas A. K. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993 Feb 1;177(2):457–463. doi: 10.1084/jem.177.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H. J., Shea C. M., Abbas A. K. Aqueous antigens induce in vivo tolerance selectively in IL-2- and IFN-gamma-producing (Th1) cells. J Immunol. 1992 Jun 15;148(12):3687–3691. [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner C., Szépfalusi Z., Ferreira F., Jilek A., Valenta R., Parronchi P., Maggi E., Romagnani S., Scheiner O., Kraft D. Identification of multiple T cell epitopes on Bet v I, the major birch pollen allergen, using specific T cell clones and overlapping peptides. J Immunol. 1993 Feb 1;150(3):1047–1054. [PubMed] [Google Scholar]
- Evavold B. D., Allen P. M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991 May 31;252(5010):1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Lancki D. W., Stack R., Fitch F. W. "Anergy" of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994 Feb 1;179(2):481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhlin L., Johansson G. O., Bennich H., Högman C., Thyresson N. Immunoglobulin E in dermatoses. Levels in atopic dermatitis and urticaria. Arch Dermatol. 1969 Jul;100(1):12–16. doi: 10.1001/archderm.100.1.12. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., Steinman R. M. Clonal expansion of human T lymphocytes initiated by dendritic cells. J Exp Med. 1989 Jan 1;169(1):315–320. doi: 10.1084/jem.169.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKie R. M., Cobb S. J., Cochran R. E., Thomson J. Total and specific IgE levels in patients with atopic dermatitis. The correlation between prick testing, clinical history of allergy, and in vitro quantification of IgE during clinical exacerbation and remission. Clin Exp Dermatol. 1979 Jun;4(2):187–195. doi: 10.1111/j.1365-2230.1979.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mudde G. C., Hansel T. T., von Reijsen F. C., Osterhoff B. F., Bruijnzeel-Koomen C. A. IgE: an immunoglobulin specialized in antigen capture? Immunol Today. 1990 Dec;11(12):440–443. doi: 10.1016/0167-5699(90)90172-6. [DOI] [PubMed] [Google Scholar]
- Mudde G. C., Van Reijsen F. C., Boland G. J., de Gast G. C., Bruijnzeel P. L., Bruijnzeel-Koomen C. A. Allergen presentation by epidermal Langerhans' cells from patients with atopic dermatitis is mediated by IgE. Immunology. 1990 Mar;69(3):335–341. [PMC free article] [PubMed] [Google Scholar]
- Mueller D. L., Chiodetti L., Bacon P. A., Schwartz R. H. Clonal anergy blocks the response to IL-4, as well as the production of IL-2, in dual-producing T helper cell clones. J Immunol. 1991 Dec 15;147(12):4118–4125. [PubMed] [Google Scholar]
- O'Hehir R. E., Lamb J. R. Human in vitro experimental model for CD4+ T cell targeted immunotherapy to house dust mite. Ann Allergy. 1993 Sep;71(3):317–321. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Ramb-Lindhauer C., Feldmann A., Rotte M., Neumann C. Characterization of grass pollen reactive T-cell lines derived from lesional atopic skin. Arch Dermatol Res. 1991;283(2):71–76. doi: 10.1007/BF00371611. [DOI] [PubMed] [Google Scholar]
- Reinhold U., Kukel S., Goeden B., Neumann U., Wehrmann W., Kreysel H. W. Interleukin-4 promotes the expansion of skin-infiltrating lymphocytes from atopic dermatitis in vitro. J Invest Dermatol. 1991 Mar;96(3):370–375. doi: 10.1111/1523-1747.ep12466152. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager N., Feldmann A., Schilling G., Kreitsch P., Neumann C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J Allergy Clin Immunol. 1992 Apr;89(4):801–810. doi: 10.1016/0091-6749(92)90434-4. [DOI] [PubMed] [Google Scholar]
- Santamaria L. F., Bheekha R., van Reijsen F. C., Perez Soler M. T., Suter M., Bruijnzeel-Koomen C. A., Mudde G. C. Antigen focusing by specific monomeric immunoglobulin E bound to CD23 on Epstein-Barr virus-transformed B cells. Hum Immunol. 1993 May;37(1):23–30. doi: 10.1016/0198-8859(93)90139-r. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Evavold B. D., Allen P. M. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993 May 13;363(6425):156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Strange P., Cooper K. D., Hansen E. R., Fisher G., Larsen J. K., Fox D., Krag C., Voorhees J. J., Baadsgaard O. T-lymphocyte clones initiated from lesional psoriatic skin release growth factors that induce keratinocyte proliferation. J Invest Dermatol. 1993 Nov;101(5):695–700. doi: 10.1111/1523-1747.ep12371678. [DOI] [PubMed] [Google Scholar]
- Symington F. W., Brady W., Linsley P. S. Expression and function of B7 on human epidermal Langerhans cells. J Immunol. 1993 Feb 15;150(4):1286–1295. [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., Bos J. D., Jansen H. M., Kapsenberg M. L. Comparison of diversity and function of house dust mite-specific T lymphocyte clones from atopic and non-atopic donors. Eur J Immunol. 1990 Jul;20(7):1519–1526. doi: 10.1002/eji.1830200717. [DOI] [PubMed] [Google Scholar]
- Zubiaga A. M., Munoz E., Huber B. T. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992 Jul 1;149(1):107–112. [PubMed] [Google Scholar]
- van Reijsen F. C., Bruijnzeel-Koomen C. A., Kalthoff F. S., Maggi E., Romagnani S., Westland J. K., Mudde G. C. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992 Aug;90(2):184–193. doi: 10.1016/0091-6749(92)90070-i. [DOI] [PubMed] [Google Scholar]
- van der Heijden F. L., Joost van Neerven R. J., van Katwijk M., Bos J. D., Kapsenberg M. L. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993 Apr 15;150(8 Pt 1):3643–3650. [PubMed] [Google Scholar]
- van der Heijden F. L., Wierenga E. A., Bos J. D., Kapsenberg M. L. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991 Sep;97(3):389–394. doi: 10.1111/1523-1747.ep12480966. [DOI] [PubMed] [Google Scholar]


