Abstract
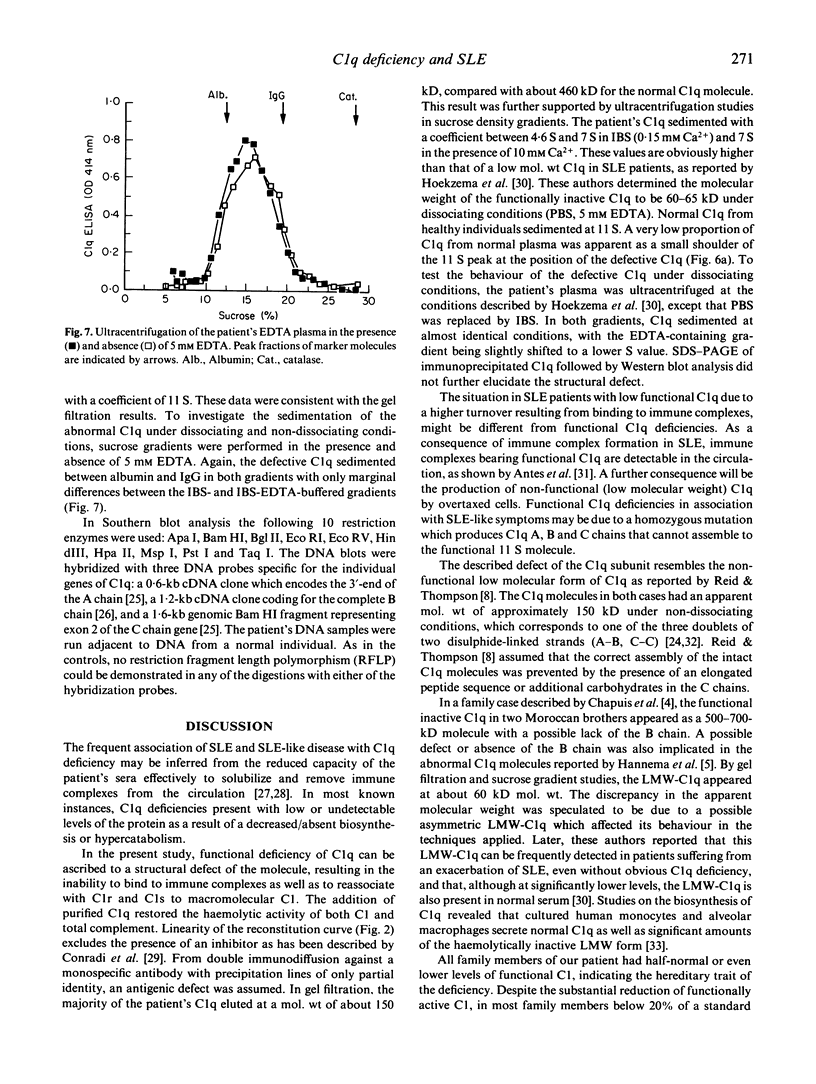
A complete functional deficiency of C1q is described in a patient suffering from SLE. From reduced plasma C1 activity of the parents a hereditary trait was assumed. The defective C1q molecule was haemolytically inactive, did not bind to immune complexes, and was not recognized by the monocyte C1q receptor. C1 activity in the patient's serum could be restored by the addition of purified C1q. Analysis by gel-filtration and ultracentrifugation experiments revealed an immunoreactive molecule of about 150 kD mol. wt, corresponding to one structural subunit of the C1q macromolecule, containing two A chain-B chain dimers and a C-C chain dimer. Applying Southern blot analysis with cDNA clones encoding for the three individual chains of the C1q molecule, no restriction fragment length polymorphism was detected, ruling out possible major alterations of the genetic information.
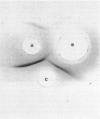
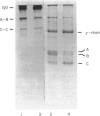
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antes U., Heinz H. P., Loos M. Detection of C1q-bearing immune complexes by a monoclonal anti-C1q ELISA system. J Immunol Methods. 1987 Sep 24;102(2):149–156. doi: 10.1016/0022-1759(87)90071-8. [DOI] [PubMed] [Google Scholar]
- Antes U., Heinz H. P., Loos M. Enzyme-linked immunosorbent assay for C1q in human serum by use of monoclonal antibodies. J Immunol Methods. 1984 Nov 30;74(2):299–306. doi: 10.1016/0022-1759(84)90297-7. [DOI] [PubMed] [Google Scholar]
- Antes U., Heinz H. P., Loos M. Evidence for the presence of autoantibodies to the collagen-like portion of C1q in systemic lupus erythematosus. Arthritis Rheum. 1988 Apr;31(4):457–464. doi: 10.1002/art.1780310401. [DOI] [PubMed] [Google Scholar]
- Borsos T., Loos M., Chapuis R. M., Medicus R., Isliker H. A novel way of relating the structure of Cl1 to the hemolytic activity of the first component of complement. Mol Immunol. 1980 Nov;17(11):1415–1421. doi: 10.1016/0161-5890(80)90011-5. [DOI] [PubMed] [Google Scholar]
- Brown E. J. An improved method for performance of the C1 fixation and transfer test. J Immunol Methods. 1982 Nov 26;55(1):115–122. doi: 10.1016/0022-1759(82)90084-9. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of human blood monocytes with Nycodenz, a new non-ionic iodinated gradient medium. Scand J Immunol. 1983 May;17(5):429–436. doi: 10.1111/j.1365-3083.1983.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Chapuis R. M., Hauptmann G., Grosshans E., Isliker H. Structural and functional studies in C1q deficiency. J Immunol. 1982 Oct;129(4):1509–1512. [PubMed] [Google Scholar]
- Conradie J. D., Volanakis J. E., Stroud R. M. Evidence for a serum inhibitor of Clq. Immunochemistry. 1975 Dec;12(12):967–971. doi: 10.1016/0019-2791(75)90260-8. [DOI] [PubMed] [Google Scholar]
- Hannema A. J., Kluin-Nelemans J. C., Hack C. E., Eerenberg-Belmer A. J., Mallée C., van Helden H. P. SLE like syndrome and functional deficiency of C1q in members of a large family. Clin Exp Immunol. 1984 Jan;55(1):106–114. [PMC free article] [PubMed] [Google Scholar]
- Hoekzema R., Brouwer M. C., de Graeff-Meeder E. R., van Helden H. P., Hack C. E. Biosynthesis of normal and low-molecular-mass complement component C1q by cultured human monocytes and macrophages. Biochem J. 1989 Jan 15;257(2):477–486. doi: 10.1042/bj2570477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema R., Hannema A. J., Swaak T. J., Paardekooper J., Hack C. E. Low molecular weight C1q in systemic lupus erythematosus. J Immunol. 1985 Jul;135(1):265–271. [PubMed] [Google Scholar]
- Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987 Nov 25;15(22):9611–9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hawiger A., Gelfand J. A. A study of optimal reaction conditions for an assay of the human alternative complement pathway. Am J Clin Pathol. 1983 Jan;79(1):65–72. doi: 10.1093/ajcp/79.1.65. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langone J. J., Boyle M. D., Borsos T. 125I protein A: applications to the quantitative determination of fluid phase and cell-bound IgG. J Immunol Methods. 1977;18(3-4):281–293. doi: 10.1016/0022-1759(77)90182-x. [DOI] [PubMed] [Google Scholar]
- McAdam R. A., Goundis D., Reid K. B. A homozygous point mutation results in a stop codon in the C1q B-chain of a C1q-deficient individual. Immunogenetics. 1988;27(4):259–264. doi: 10.1007/BF00376120. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Walport M. J. Complement deficiency and disease. Immunol Today. 1991 Sep;12(9):301–306. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Molecular cloning and characterization of the complementary DNA and gene coding for the B-chain of subcomponent C1q of the human complement system. Biochem J. 1985 Nov 1;231(3):729–735. doi: 10.1042/bj2310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Thompson R. A. Characterization of a non-functional form of C1q found in patients with a genetically linked deficiency of C1q activity. Mol Immunol. 1983 Oct;20(10):1117–1125. doi: 10.1016/0161-5890(83)90121-9. [DOI] [PubMed] [Google Scholar]
- Schifferli J. A., Ng Y. C., Peters D. K. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986 Aug 21;315(8):488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- Sellar G. C., Blake D. J., Reid K. B. Characterization and organization of the genes encoding the A-, B- and C-chains of human complement subcomponent C1q. The complete derived amino acid sequence of human C1q. Biochem J. 1991 Mar 1;274(Pt 2):481–490. doi: 10.1042/bj2740481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Identification of types of cells in human peripheral blood that bind C1q. J Immunol. 1981 Mar;126(3):1174–1179. [PubMed] [Google Scholar]
- Thompson R. A., Haeney M., Reid K. B., Davies J. G., White R. H., Cameron A. H. A genetic defect of the C1q subcomponent of complement associated with childhood (immune complex) nephritis. N Engl J Med. 1980 Jul 3;303(1):22–24. doi: 10.1056/NEJM198007033030107. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]