Abstract
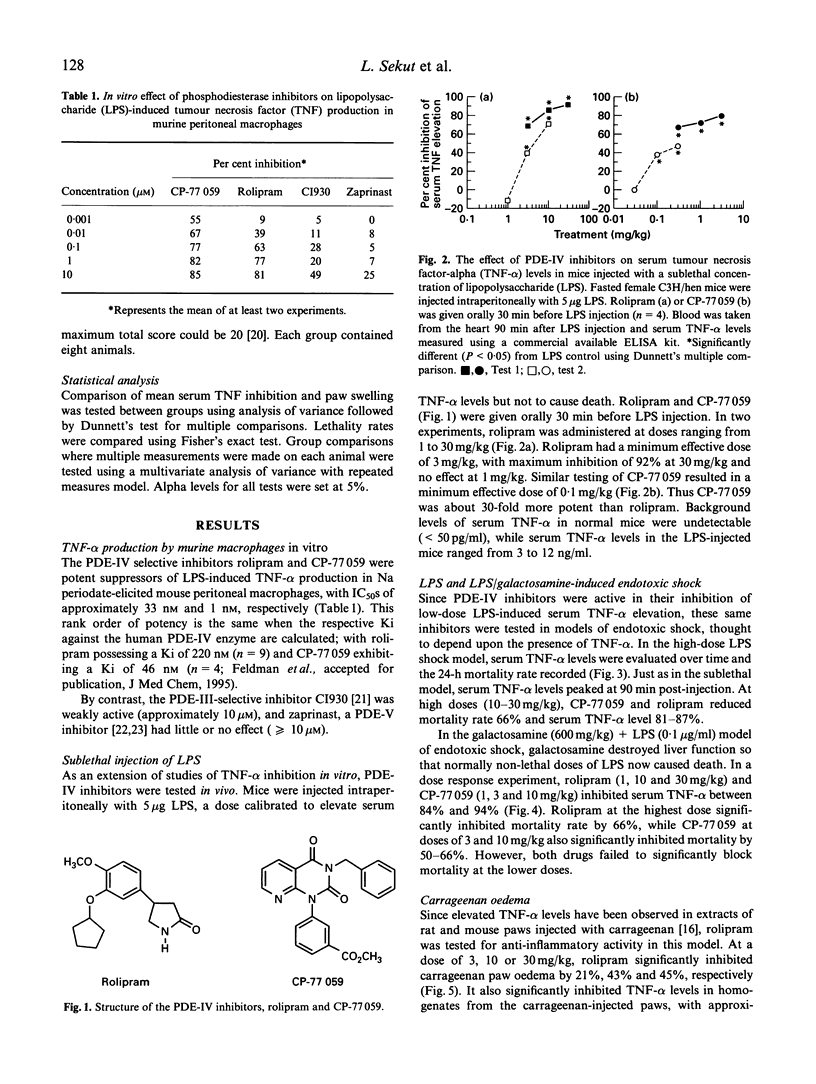
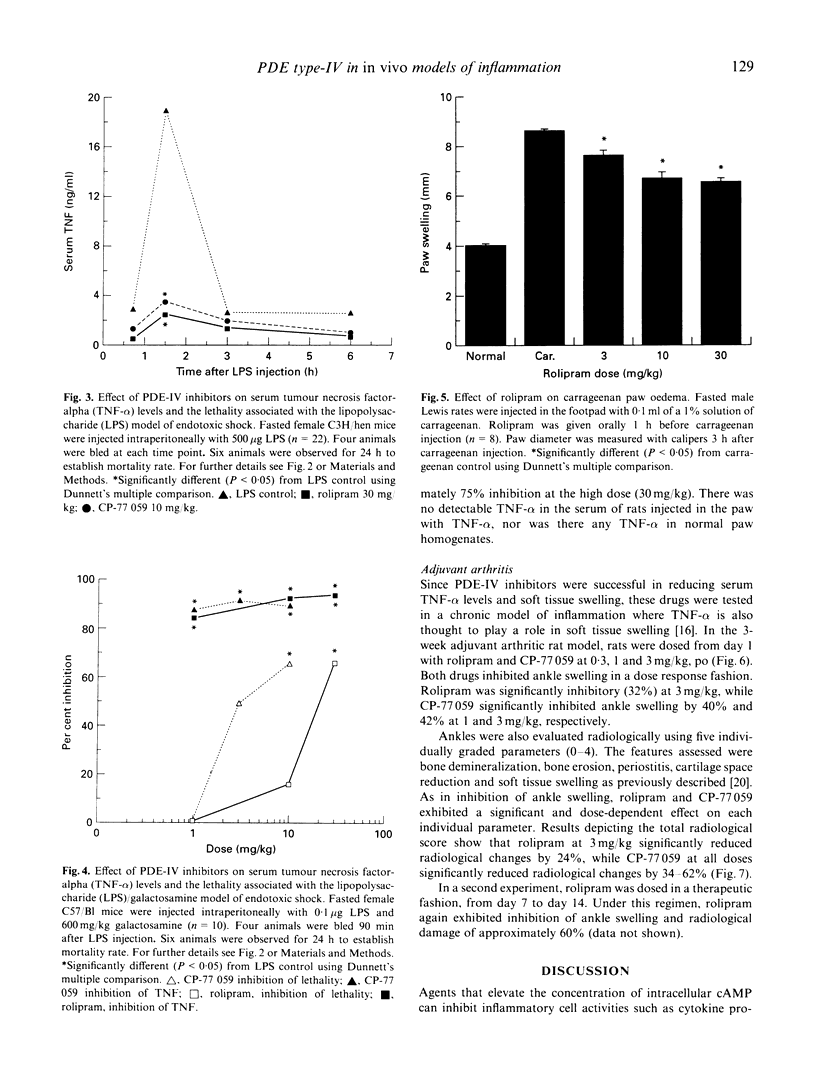
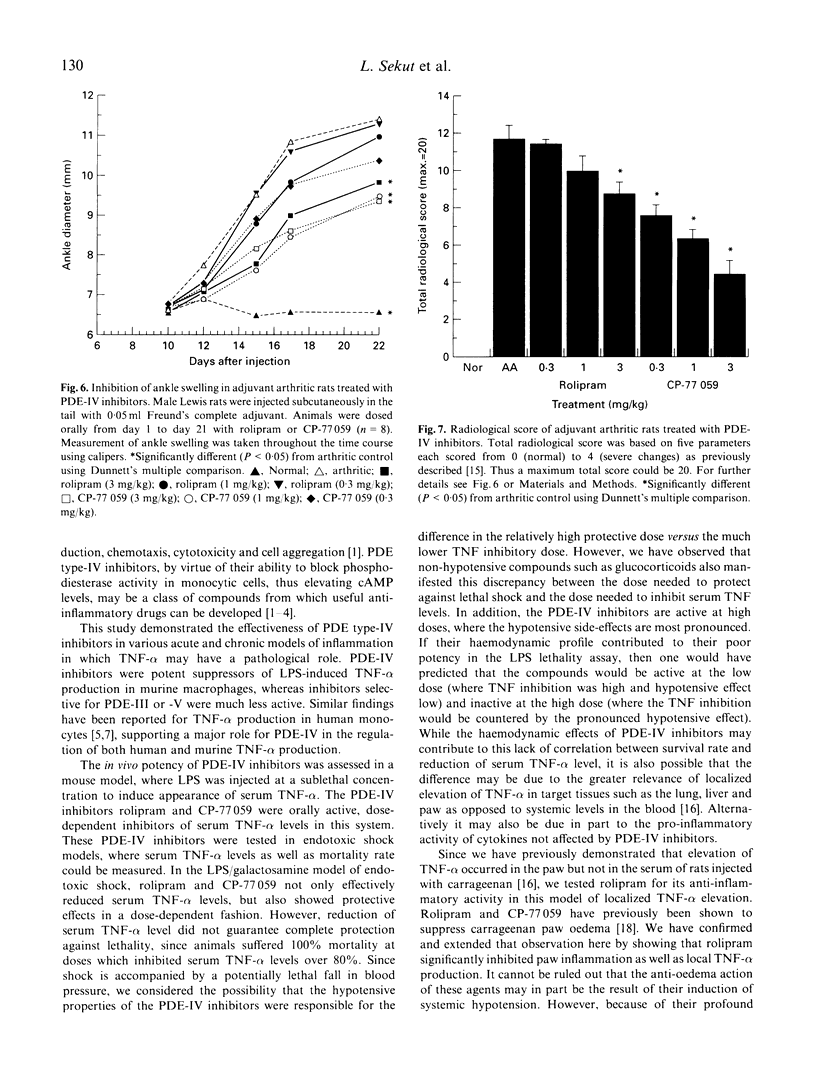
Inhibitors of cyclic nucleotide phosphodiesterases are known to suppress lipopolysaccharide (LPS)-induced tumour necrosis factor-alpha (TNF-alpha) production in vitro in human monocytes. The most potent of these have selectivity for type IV PDEs, suggesting that this class of PDE is the major type involved in the regulation of human TNF-alpha production. Using compounds of two distinct chemical structural classes, a quinazolinedione (CP-77059) and a 4 arylpyrrolidinone (rolipram), we show here that PDE-IV-specific inhibitors are also potent in suppressing LPS-induced TNF-alpha production in vitro in sodium periodate-elicited murine macrophages (IC50s of 1 and 33, respectively). We then report the in vivo anti-inflammatory effect of PDE-IV inhibition in five murine models of inflammation: (i) elevation of serum TNF-alpha induced by a sublethal LPS injection; (ii) LPS-induced endotoxic shock; (iii) LPS/galactosamine-induced endotoxic shock; (iv) carrageenan-induced paw oedema; and (v) adjuvant arthritis. Following a sublethal (5 micrograms/mouse) injection of LPS, serum TNF-alpha levels in mice peaked sharply, reaching concentrations of 3-12 ng/ml 90 min after injection. In this sublethal LPS assay, CP-77059 was about 30 times more potent than rolipram, with a minimum effective dose of 0.1 mg/kg versus 3 mg/kg for rolipram. This rank order is in keeping with the relative in vitro IC50s for CP-77059 and rolipram, as well as their relative Ki against the human PDE-IV enzyme (46 nM and 220 nM, respectively). In LPS-induced endotoxic shock, rolipram and CP-77059 at relatively high doses of 30 and 10 mg/kg, respectively, significantly reduced serum TNF-alpha levels, and also inhibited mortality 66%. In the LPS/galactosamine shock model, in which mice are rendered exquisitely sensitive to LPS by co-injection with galactosamine, only 0.1 microgram of LPS/mouse is necessary for serum TNF-alpha elevation and death. Both rolipram and the CP-77059 caused dose-dependent reduction of serum TNF-alpha and lethality. In the carrageenan-induced paw oedema model, in which there is a pronounced local TNF-alpha response (without a serum TNF-alpha elevation), rolipram significantly inhibited paw swelling as well as localized TNF-alpha levels in the paw. In the adjuvant arthritis model, a chronic model of inflammation also possessing localized TNF-alpha elevation in the inflamed paw, rolipram and CP-77059 suppressed ankle swelling and radiological evidence of joint damage. These data are consistent with a major role for PDE-IV in regulation of TNF-alpha production and inflammatory responses in murine systems.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Cuttino J. T., Jr, Anderle S. K., Cromartie W. J., Schwab J. H. Radiologic analysis of arthritis in rats after systemic injection of streptococcal cell walls. Arthritis Rheum. 1979 Jan;22(1):25–35. doi: 10.1002/art.1780220105. [DOI] [PubMed] [Google Scholar]
- Connolly K. M., Stecher V. J., Kent L. Examination of interleukin-1 activity, the acute phase response, and leukocyte subpopulations in rats with adjuvant-induced arthritis. J Lab Clin Med. 1988 Mar;111(3):341–347. [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Giembycz M. A., Dent G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin Exp Allergy. 1992 Mar;22(3):337–344. doi: 10.1111/j.1365-2222.1992.tb03095.x. [DOI] [PubMed] [Google Scholar]
- Griswold D. E., Webb E. F., Breton J., White J. R., Marshall P. J., Torphy T. J. Effect of selective phosphodiesterase type IV inhibitor, rolipram, on fluid and cellular phases of inflammatory response. Inflammation. 1993 Jun;17(3):333–344. doi: 10.1007/BF00918994. [DOI] [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Livi G. P., Kmetz P., McHale M. M., Cieslinski L. B., Sathe G. M., Taylor D. P., Davis R. L., Torphy T. J., Balcarek J. M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol Cell Biol. 1990 Jun;10(6):2678–2686. doi: 10.1128/mcb.10.6.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J. A., 3rd, Archer R. L., Chapin D. S., Chen J. B., Helweg D., Johnson J. L., Koe B. K., Lebel L. A., Moore P. F., Nielsen J. A. Structure-activity relationship of quinazolinedione inhibitors of calcium-independent phosphodiesterase. J Med Chem. 1991 Feb;34(2):624–628. doi: 10.1021/jm00106a024. [DOI] [PubMed] [Google Scholar]
- Lowe M. E. Site-specific mutations in the COOH-terminus of placental alkaline phosphatase: a single amino acid change converts a phosphatidylinositol-glycan-anchored protein to a secreted protein. J Cell Biol. 1992 Feb;116(3):799–807. doi: 10.1083/jcb.116.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M. M., Cieslinski L. B., Burman M., Torphy T. J., Livi G. P. A low-Km, rolipram-sensitive, cAMP-specific phosphodiesterase from human brain. Cloning and expression of cDNA, biochemical characterization of recombinant protein, and tissue distribution of mRNA. J Biol Chem. 1993 Mar 25;268(9):6470–6476. [PubMed] [Google Scholar]
- Nielson C. P., Vestal R. E., Sturm R. J., Heaslip R. Effects of selective phosphodiesterase inhibitors on the polymorphonuclear leukocyte respiratory burst. J Allergy Clin Immunol. 1990 Nov;86(5):801–808. doi: 10.1016/s0091-6749(05)80186-1. [DOI] [PubMed] [Google Scholar]
- Nielson C. P., Vestal R. E., Sturm R. J., Heaslip R. Effects of selective phosphodiesterase inhibitors on the polymorphonuclear leukocyte respiratory burst. J Allergy Clin Immunol. 1990 Nov;86(5):801–808. doi: 10.1016/s0091-6749(05)80186-1. [DOI] [PubMed] [Google Scholar]
- Peachell P. T., Undem B. J., Schleimer R. P., MacGlashan D. W., Jr, Lichtenstein L. M., Cieslinski L. B., Torphy T. J. Preliminary identification and role of phosphodiesterase isozymes in human basophils. J Immunol. 1992 Apr 15;148(8):2503–2510. [PubMed] [Google Scholar]
- Peachell P. T., Undem B. J., Schleimer R. P., MacGlashan D. W., Jr, Lichtenstein L. M., Cieslinski L. B., Torphy T. J. Preliminary identification and role of phosphodiesterase isozymes in human basophils. J Immunol. 1992 Apr 15;148(8):2503–2510. [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Vassalli P. Tumor necrosis factor and immunopathology. Immunol Res. 1991;10(2):122–140. doi: 10.1007/BF02918160. [DOI] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Robicsek S. A., Blanchard D. K., Djeu J. Y., Krzanowski J. J., Szentivanyi A., Polson J. B. Multiple high-affinity cAMP-phosphodiesterases in human T-lymphocytes. Biochem Pharmacol. 1991 Jul 25;42(4):869–877. doi: 10.1016/0006-2952(91)90047-9. [DOI] [PubMed] [Google Scholar]
- Sekut L., Champion B. R., Page K., Menius J. A., Jr, Connolly K. M. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol. 1995 Mar;99(3):461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekut L., Menius J. A., Jr, Brackeen M. F., Connolly K. M. Evaluation of the significance of elevated levels of systemic and localized tumor necrosis factor in different animal models of inflammation. J Lab Clin Med. 1994 Dec;124(6):813–820. [PubMed] [Google Scholar]
- Semmler J., Wachtel H., Endres S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int J Immunopharmacol. 1993 Apr;15(3):409–413. doi: 10.1016/0192-0561(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Smith-Oliver T., Noel L. S., Stimpson S. S., Yarnall D. P., Connolly K. M. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine. 1993 Jul;5(4):298–304. doi: 10.1016/1043-4666(93)90060-i. [DOI] [PubMed] [Google Scholar]
- Torphy T. J., Undem B. J. Phosphodiesterase inhibitors: new opportunities for the treatment of asthma. Thorax. 1991 Jul;46(7):512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]


