Abstract
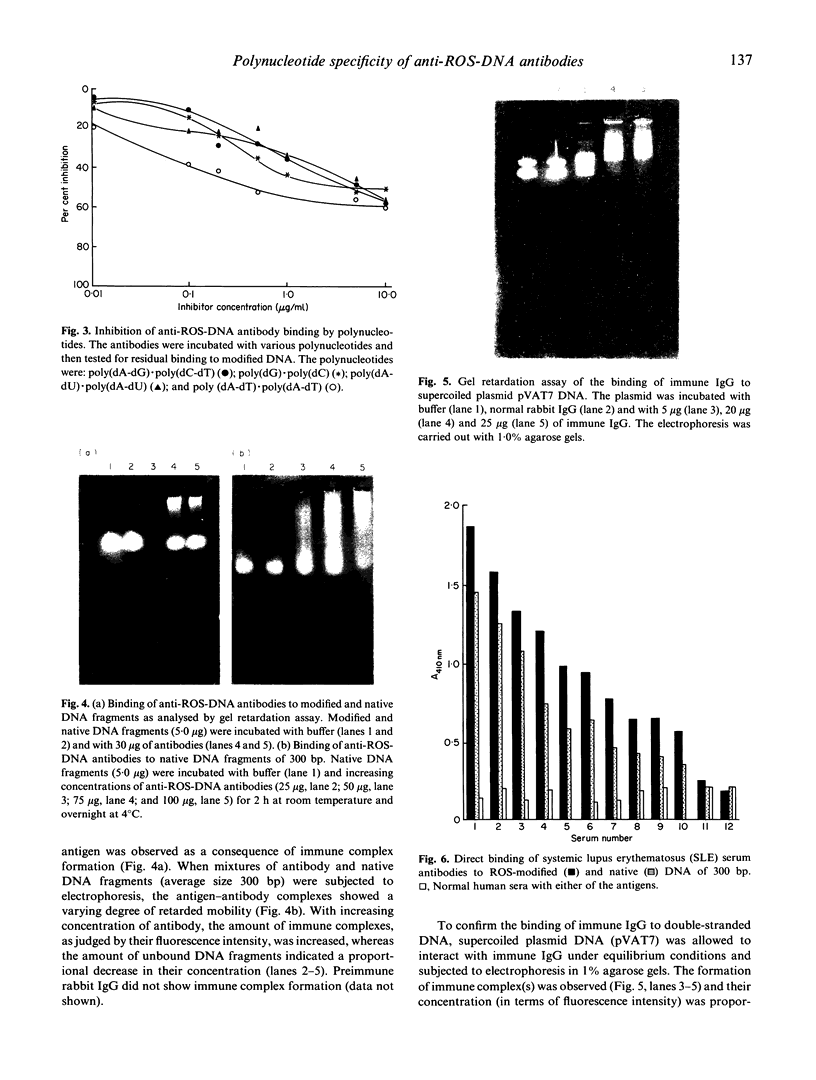
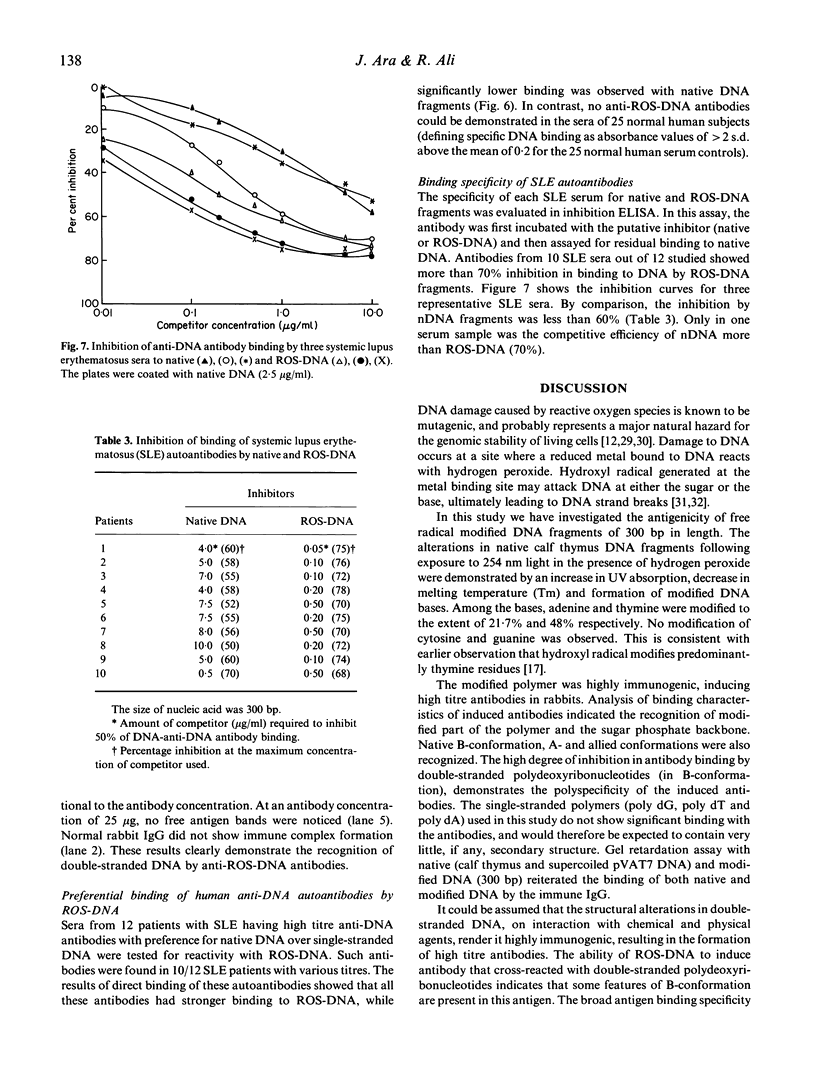
Hydrogen peroxide in the presence of short wavelength UV light was able to induce alterations in native DNA fragments of 300 bp (ROS-DNA), thereby rendering it immunogenic in experimental animals. The specificity of induced antibodies was investigated by direct binding and competition ELISA. Inhibition studies revealed nearly 89% inhibition in the antibody binding by the immunogen and recognition of native B-, A- and allied conformations presented by various synthetic polynucleotides. Gel retardation assay reiterated the formation of immune complexes between induced antibodies and native and ROS-DNA fragments. It was observed that naturally occurring anti-DNA autoantibodies from systemic lupus erythematosus (SLE) sera recognize ROS-DNA. The comparison of the specificities of anti-DNA autoantibodies from 10 SLE patients showed a 20-50-fold preference for ROS-DNA over native DNA. These results demonstrate that anti-DNA antibodies can be induced by ROS-DNA, and that some of the autoimmune DNA binding antibodies found in SLE may result from response to reactive oxygen species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam K., Ali R. Human autoantibody binding to multiple conformations of DNA. Biochem Int. 1992 Mar;26(4):597–605. [PubMed] [Google Scholar]
- Ali R., Dersimonian H., Stollar B. D. Binding of monoclonal anti-native DNA autoantibodies to DNA of varying size and conformation. Mol Immunol. 1985 Dec;22(12):1415–1422. doi: 10.1016/0161-5890(85)90065-3. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Anderson W. F., Cygler M., Braun R. P., Lee J. S. Antibodies to DNA. Bioessays. 1988 Feb-Mar;8(2):69–74. doi: 10.1002/bies.950080206. [DOI] [PubMed] [Google Scholar]
- Aotsuka S., Okawa M., Ikebe K., Yokohari R. Measurement of anti-double-stranded DNA antibodies in major immunoglobulin classes. J Immunol Methods. 1979;28(1-2):149–162. doi: 10.1016/0022-1759(79)90337-5. [DOI] [PubMed] [Google Scholar]
- Ara J., Ali A., Ali R. Antibodies against free radical modified native DNA recognize B-conformation. Immunol Invest. 1992 Oct;21(6):553–563. doi: 10.3109/08820139209069390. [DOI] [PubMed] [Google Scholar]
- Ara J., Ali R. Reactive oxygen species modified DNA fragments of varying size are the preferred antigen for human anti-DNA autoantibodies. Immunol Lett. 1992 Dec;34(3):195–200. doi: 10.1016/0165-2478(92)90213-8. [DOI] [PubMed] [Google Scholar]
- Blount S., Griffiths H. R., Lunec J. Reactive oxygen species induce antigenic changes in DNA. FEBS Lett. 1989 Mar 13;245(1-2):100–104. doi: 10.1016/0014-5793(89)80200-5. [DOI] [PubMed] [Google Scholar]
- Blount S., Griffiths H., Emery P., Lunec J. Reactive oxygen species modify human DNA, eliciting a more discriminating antigen for the diagnosis of systemic lupus erythematosus. Clin Exp Immunol. 1990 Sep;81(3):384–389. doi: 10.1111/j.1365-2249.1990.tb05343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. P., Lee J. S. Immunogenic duplex nucleic acids are nuclease resistant. J Immunol. 1988 Sep 15;141(6):2084–2089. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Hüttermann J., Lange M., Ohlmann J. Mechanistic aspects of radiation-induced free radical formation in frozen aqueous solutions of DNA constituents: consequences for DNA? Radiat Res. 1992 Jul;131(1):18–23. [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Ishaq M., Ali R. Identical specificity of lupus antibodies and antibodies elicited in rabbits against Sm and RNP antigens. Immunol Commun. 1984;13(5):447–455. doi: 10.3109/08820138409033891. [DOI] [PubMed] [Google Scholar]
- Karounos D. G., Grudier J. P., Pisetsky D. S. Spontaneous expression of antibodies to DNA of various species origin in sera of normal subjects and patients with systemic lupus erythematosus. J Immunol. 1988 Jan 15;140(2):451–455. [PubMed] [Google Scholar]
- Lewis J. G., Adams D. O. Induction of 5,6-ring-saturated thymine bases in NIH-3T3 cells by phorbol ester-stimulated macrophages: role of reactive oxygen intermediates. Cancer Res. 1985 Mar;45(3):1270–1275. [PubMed] [Google Scholar]
- Menghini R. Genotoxicity of active oxygen species in mammalian cells. Mutat Res. 1988 May;195(3):215–230. [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Rhaese H. J., Freese E. Chemical analysis of DNA alterations. I. Base liberation and backbone breakage of DNA and oligodeoxyadenylic acid induced by hydrogen peroxide and hydroxylamine. Biochim Biophys Acta. 1968 Feb 26;155(2):476–490. [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Antibodies to DNA. CRC Crit Rev Biochem. 1986;20(1):1–36. doi: 10.3109/10409238609115899. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. Immunochemistry of DNA. Int Rev Immunol. 1989;5(1):1–22. doi: 10.3109/08830188909086987. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Evans J. W., Limoli C. L., Calabro-Jones P. M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br J Cancer Suppl. 1987 Jun;8:105–112. [PMC free article] [PubMed] [Google Scholar]