Abstract
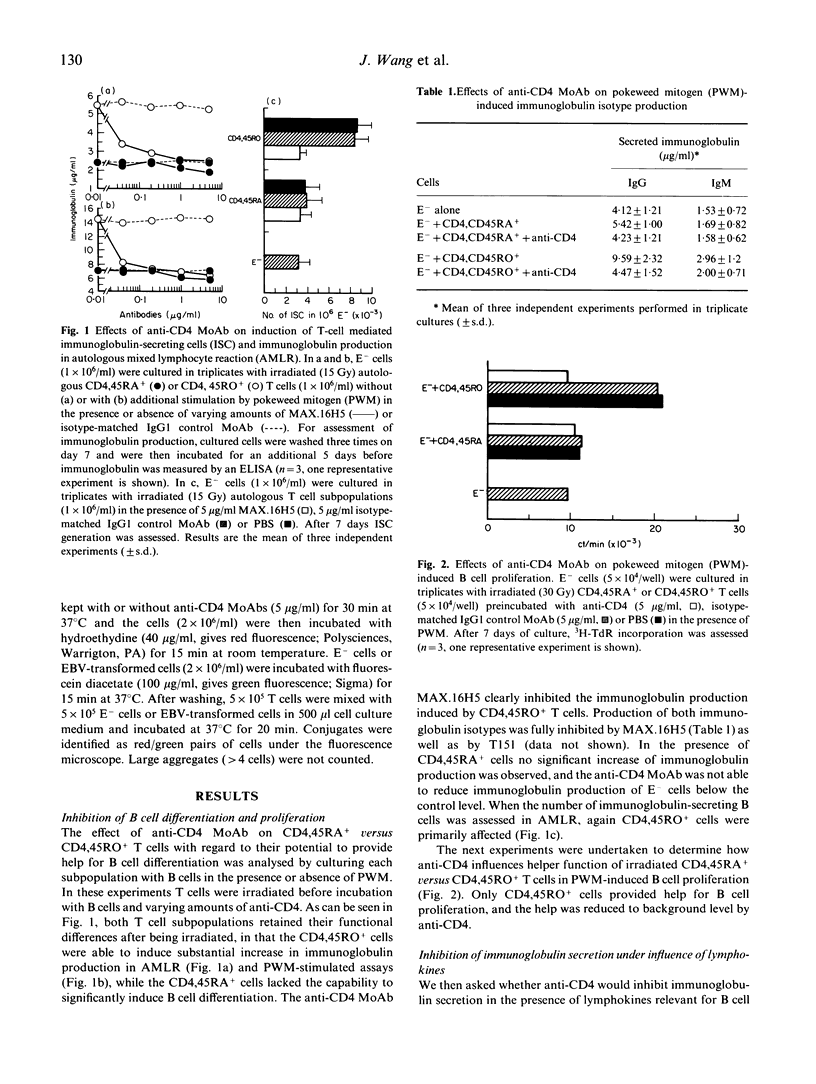
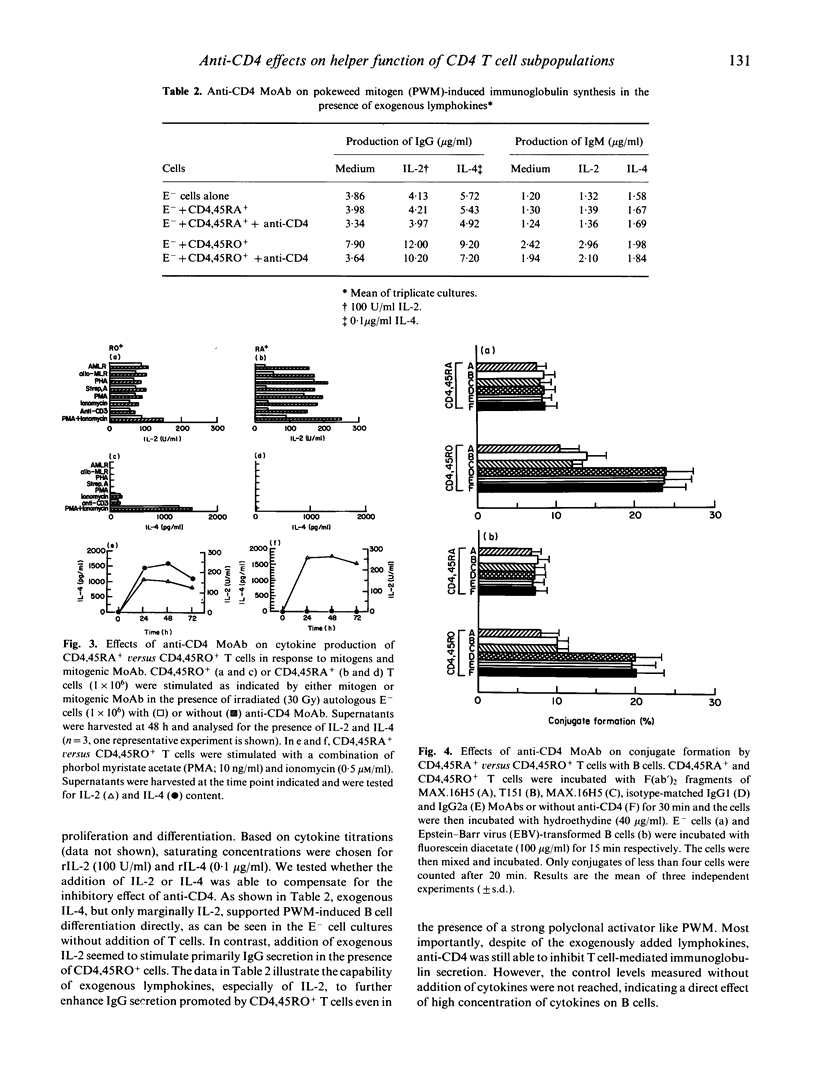
Here we have investigated and compared the effects of anti-CD4 on helper function of CD4,45RA+ versus CD4,45RO+ T cells. Only CD4,45RO+ cells, but not CD4,45RA+ cells were able to promote B cell differentiation resulting in immunoglobulin production in vitro (IgM as well as IgG) which could be inhibited by anti-CD4 MoAbs (MAX.16H5 and T151). In pokeweed mitogen (PWM)-induced B cell proliferation a similar pattern of responsiveness was obtained. When we studied the anti-CD4 effects on cytokine production in T cells stimulated in mixed lymphocyte reaction (MLR) or by mitogens, we found that neither IL-2 nor IL-4 production was dramatically influenced by anti-CD4 in CD4,45RO+ cells. This led us to the conclusion that the inhibitory effect of anti-CD4 on B cell proliferation and immunoglobulin secretion was not due to inhibition of cytokine production. To clarify this point, we investigated the ability of anti-CD4 to inhibit conjugate formation between B and T cells. It was found that CD4,45RO+ T cells formed more conjugates than CD4,45RA+ cells, and that only the conjugate formation by CD4,45RO+ T cells was inhibited by anti-CD4. These results suggest that (i) anti-CD4 inhibits T helper functions primarily by affecting CD4,45RO+ cells, and (ii) this effect is probably mediated by contact inhibition in the early phase of T-B collaboration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M., Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991 Jun;12(6):184–188. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- Andersson U., Andersson J., Lindfors A., Wagner K., Möller G., Heusser C. H. Simultaneous production of interleukin 2, interleukin 4 and interferon-gamma by activated human blood lymphocytes. Eur J Immunol. 1990 Jul;20(7):1591–1596. doi: 10.1002/eji.1830200727. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Bettens F., Walker C., Gauchat J. F., Gauchat D., Wyss T., Pichler W. J. Lymphokine gene expression related to CD4 T cell subset (CD45R/CDw29) phenotype conversion. Eur J Immunol. 1989 Sep;19(9):1569–1574. doi: 10.1002/eji.1830190908. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Butler J. L., Reinherz E. L., Cooper M. D. Virgin and memory T cells have different requirements for activation via the CD2 molecule. Int Immunol. 1989;1(1):29–35. doi: 10.1093/intimm/1.1.29. [DOI] [PubMed] [Google Scholar]
- Dianzani U., Luqman M., Rojo J., Yagi J., Baron J. L., Woods A., Janeway C. A., Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunological memory. Eur J Immunol. 1990 Oct;20(10):2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Horneff G., Becker W., Lüke W., Potocnik A., Kanzy U., Kalden J. R., Burmester G. An anti-CD4 antibody for treatment of chronic inflammatory arthritis. Agents Actions Suppl. 1991;32:165–170. doi: 10.1007/978-3-0348-7405-2_22. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Moll H., Simon M. M. Recombinant human interleukin 2 acts as a B cell growth and differentiation promoting factor. Immunobiology. 1985 Feb;169(1):97–102. doi: 10.1016/S0171-2985(85)80057-7. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Schilling B., Eichmann K. Human immune response to group A streptococcal carbohydrate (A-CHO). I. Quantitative and qualitative analysis of the A-CHO-specific B cell population responding in vitro to polyclonal and specific activation. J Exp Med. 1985 Mar 1;161(3):547–562. doi: 10.1084/jem.161.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Okumura M., Inada K., Nakahara K., Matsuda H. CD45 isoform expression during T cell development in the thymus. Eur J Immunol. 1992 Jul;22(7):1843–1850. doi: 10.1002/eji.1830220725. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Weiner H. L. Immunosuppression with monoclonal antibodies in multiple sclerosis. Neurology. 1988 Jul;38(7 Suppl 2):42–47. [PubMed] [Google Scholar]
- Herzog C., Walker C., Pichler W., Aeschlimann A., Wassmer P., Stockinger H., Knapp W., Rieber P., Müller W. Monoclonal anti-CD4 in arthritis. Lancet. 1987 Dec 19;2(8573):1461–1462. doi: 10.1016/s0140-6736(87)91158-5. [DOI] [PubMed] [Google Scholar]
- Hiepe F., Volk H. D., Apostoloff E., von Baehr R., Emmrich F. Treatment of severe systemic lupus erythematosus with anti-CD4 monoclonal antibody. Lancet. 1991 Dec 14;338(8781):1529–1530. doi: 10.1016/0140-6736(91)92353-4. [DOI] [PubMed] [Google Scholar]
- Hirohata S., Jelinek D. F., Lipsky P. E. T cell-dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J Immunol. 1988 Jun 1;140(11):3736–3744. [PubMed] [Google Scholar]
- Hirohata S., Lipsky P. E. T cell regulation of human B cell proliferation and differentiation. Regulatory influences of CD45R+ and CD45R- T4 cell subsets. J Immunol. 1989 Apr 15;142(8):2597–2607. [PubMed] [Google Scholar]
- Holter W., Majdic O., Kalthoff F. S., Knapp W. Regulation of interleukin-4 production in human mononuclear cells. Eur J Immunol. 1992 Oct;22(10):2765–2767. doi: 10.1002/eji.1830221047. [DOI] [PubMed] [Google Scholar]
- Horneff G., Burmester G. R., Emmrich F., Kalden J. R. Treatment of rheumatoid arthritis with an anti-CD4 monoclonal antibody. Arthritis Rheum. 1991 Feb;34(2):129–140. doi: 10.1002/art.1780340202. [DOI] [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Regulation of human B lymphocyte activation, proliferation, and differentiation. Adv Immunol. 1987;40:1–59. doi: 10.1016/s0065-2776(08)60237-0. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Picus J., Thomas M. L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990 Jul 5;346(6279):66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- Koulova L., Yang S. Y., Dupont B. Identification of the anti-CD3-unresponsive subpopulation of CD4+, CD45RA+ peripheral T lymphocytes. J Immunol. 1990 Oct 1;145(7):2035–2043. [PubMed] [Google Scholar]
- Lecomte O., Fischer A. Antigen-independent adhesion of CD45RA (naive) and CD45RO (memory) CD4 T cells to B cells. Int Immunol. 1992 Feb;4(2):191–196. doi: 10.1093/intimm/4.2.191. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Thompson C., Finkelman F. D., Farrar J., Schaefer M., Robb R. J. Affinity-purified interleukin 2 induces proliferation of large but not small B cells. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1518–1521. doi: 10.1073/pnas.82.5.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle R. J., Daum J., Bartlett W. C., McCann J., Shepherd D. M. Cognate interactions between helper T cells and B cells. V. Reconstitution of T helper cell function using purified plasma membranes from activated Th1 and Th2 T helper cells and lymphokines. J Immunol. 1991 Feb 15;146(4):1118–1124. [PubMed] [Google Scholar]
- Noelle R. J., Ledbetter J. A., Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992 Nov;13(11):431–433. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Shackelford D. A., Hurley T. R., Johnson P., Hyman R., Sefton B. M., Trowbridge I. S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter C., Kakavand B., Rieber E. P., Schattenkirchner M., Riethmüller G., Krüger K. Treatment of rheumatoid arthritis with monoclonal CD4 antibody M-T151. Clinical results and immunopharmacologic effects in an open study, including repeated administration. Arthritis Rheum. 1991 May;34(5):525–536. doi: 10.1002/art.1780340504. [DOI] [PubMed] [Google Scholar]
- Rogozinski L., Bass A., Glickman E., Talle M. A., Goldstein G., Wang J., Chess L., Thomas Y. The T4 surface antigen is involved in the induction of helper function. J Immunol. 1984 Feb;132(2):735–739. [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Gaston J. S., Bacon P. A. Interleukin-2 production and response by helper T-cell subsets in man. Immunology. 1988 Sep;65(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Gregory A. K., Chao C. T., Fathman C. G. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987 Jul 17;237(4812):278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Sleasman J. W., Morimoto C., Schlossman S. F., Tedder T. F. The role of functionally distinct helper T lymphocyte subpopulations in the induction of human B cell differentiation. Eur J Immunol. 1990 Jun;20(6):1357–1366. doi: 10.1002/eji.1830200623. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Sakane T., Ueda Y., Murakawa Y., Tsunematsu T. Implications for the role of cognate interactions in in vitro human B cell activation by Staphylococcus aureus Cowan I and pokeweed mitogen. J Clin Invest. 1986 Jan;77(1):294–300. doi: 10.1172/JCI112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Schlossman S. F., Morimoto C. The T4 molecule differentially regulating the activation of subpopulations of T4+ cells. J Immunol. 1987 Aug 1;139(3):665–671. [PubMed] [Google Scholar]
- Wendling D., Wijdenes J., Racadot E., Morel-Fourrier B. Therapeutic use of monoclonal anti-CD4 antibody in rheumatoid arthritis. J Rheumatol. 1991 Mar;18(3):325–327. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987 May 15;138(10):3247–3253. [PubMed] [Google Scholar]


