Abstract
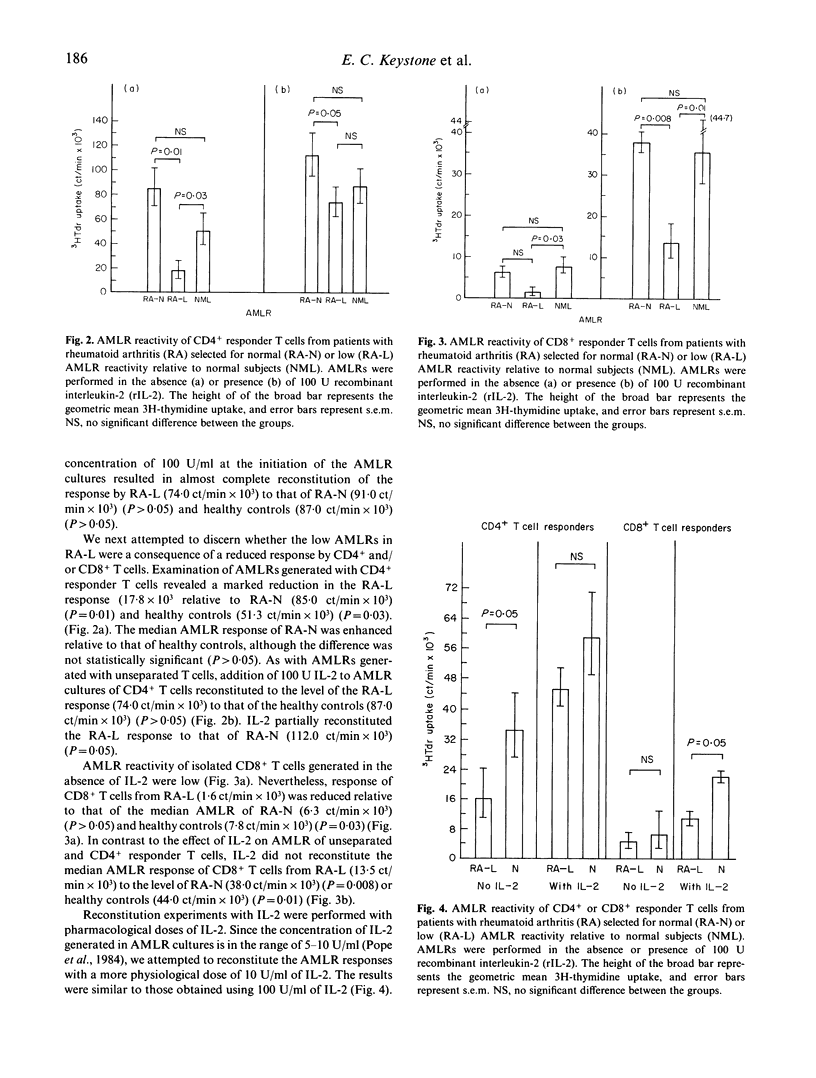
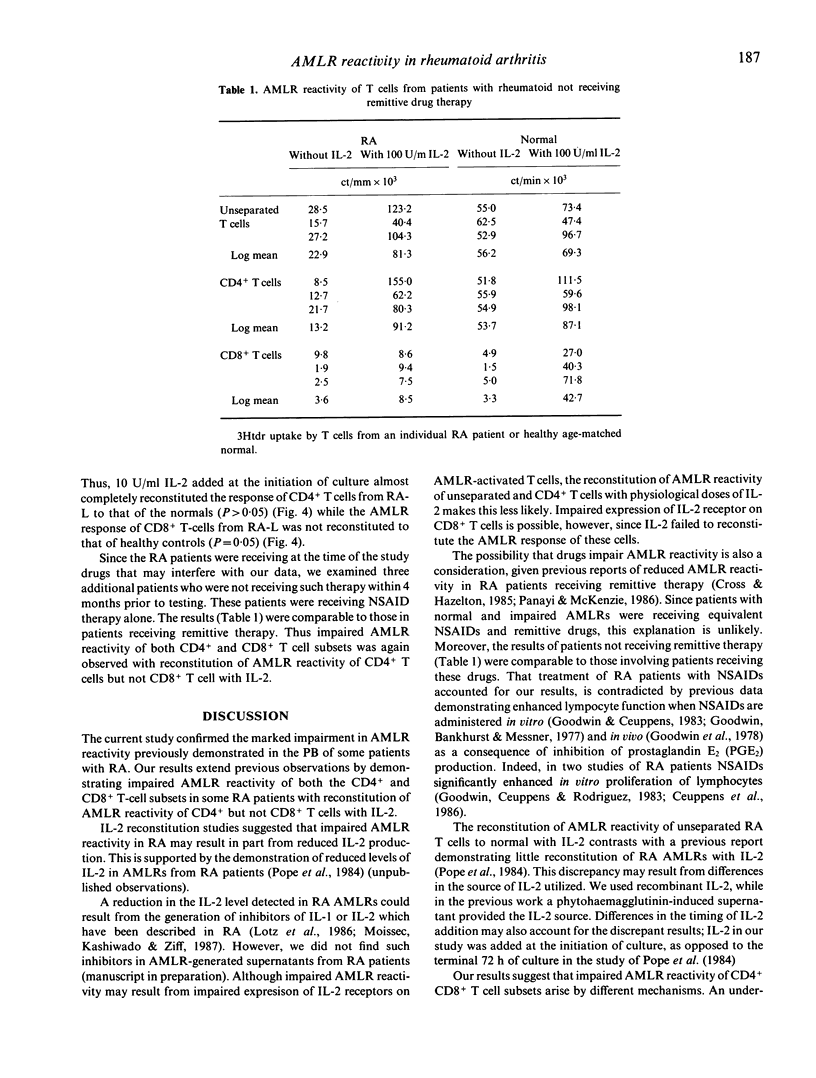
We examined AMLR reactivity of unseparated T cells and CD4+ and CD8+ T cell subsets in peripheral blood from 11 rheumatoid arthritis (RA) patients and 10 healthy controls. T cell subsets were isolated by negative selection using complement mediated cytotoxicity. AMLR reactivity of six patients (designated RA-L was reduced below the range of the controls' responses. Five patients (designated RA-N) exhibited normal AMLR reactivity. We observed impaired AMLR reactivity of CD4+ T cells from RA-L relative to RA-N and healthy controls (P < 0.05). CD4+ T cell reactivity of RA-L was reconstituted to normal with pharmacological doses of recombinant interleukin-2 (IL-2) (100 U/ml). In contrast, CD8+ T cells from RA-L in the presence of 100 U/ml IL-2 exhibited markedly impaired AMLR reactivity relative to RA-N and healthy controls (P < 0.05). Dose-response studies revealed partial reconstitution of CD4 T cells with physiological concentrations of IL-2 (10 U/ml). To examine the possibility that in vivo pre-activation of T cells in RA accounted for the findings, T cells or subsets were cultured alone for 7 days in the presence of 100 U/ml IL-2. A trend toward enhanced reactivity of CD4+ and CD8+ T cells in L-RA relative to N-RA and healthy controls was observed, but the differences were not statistically significant. There was no correlation between reactivity of T cells alone in the presence of IL-2 and AMLR reactivity. The results suggest the possibility that abnormal AMLR reactivity of CD4+ and CD8+ T cell subsets in RA may arise as a consequence of different pathophysiological mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P., Burmester G. R., Ledwoch A., Urban C., Kalden J. R. Autologous and allogeneic MLC-reactivity in patients with rheumatoid arthritis. J Clin Lab Immunol. 1981 Jul;6(1):27–33. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ceuppens J. L., Robaeys G., Verdickt W., Vertessen S., Deckmyn H., Dequeker J. Immunomodulatory effects of treatment with naproxen in patients with rheumatic disease. Arthritis Rheum. 1986 Mar;29(3):305–311. doi: 10.1002/art.1780290301. [DOI] [PubMed] [Google Scholar]
- Cross S. M., Hazelton R. A. Correlation of disease activity and drug therapy with the autologous mixed lymphocyte reaction in rheumatoid arthritis. Ann Rheum Dis. 1985 Apr;44(4):224–231. doi: 10.1136/ard.44.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis G. A., Shevach E. M. The syngeneic mixed leukocyte reaction represents polyclonal activation of antigen-specific T lymphocytes with receptors for self-Ia antigens. J Immunol. 1981 Dec;127(6):2456–2460. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Murphy S. A., Selinger D. S., Messner R. P., Williams R. C., Jr Partial reversal of the cellular immune defect in common variable immunodeficiency with indomethacin. J Clin Lab Immunol. 1978 Nov;1(3):197–199. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. L., Rodriguez M. A. Administration of nonsteroidal anti-inflammatory agents in patients with rheumatoid arthritis. Effects on indexes of cellular immune status and serum rheumatoid factor levels. JAMA. 1983 Nov 11;250(18):2485–2488. [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Uchida S. T cytotoxic and helper cells are markedly increased, and T suppressor and inducer cells are markedly decreased, in rheumatoid synovial fluids. Arthritis Rheum. 1987 Jul;30(7):737–743. doi: 10.1002/art.1780300703. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Smith K. A. Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med. 1986 Feb 1;163(2):270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C., Merkenschlager M., Gattringer C., Royston I., Fink U., Braunsteiner H. Human autologous mixed lymphocyte reactivity is primarily specific for xenoprotein determinants adsorbed to antigen-presenting cells during rosette formation with sheep erythrocytes. J Exp Med. 1982 Apr 1;155(4):1222–1227. doi: 10.1084/jem.155.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Choi Y. S. Failure of the human autologous mixed lymphocyte reaction in the absence of foreign antigens. Eur J Immunol. 1983 Dec;13(12):1031–1036. doi: 10.1002/eji.1830131215. [DOI] [PubMed] [Google Scholar]
- Kalden J. R., Leitner O., Klöckel O., Kirchner H., Manger B., Koch B. The autologous mixed lymphocyte reaction in patients with rheumatoid arthritis. Behring Inst Mitt. 1983 May;(72):193–202. [PubMed] [Google Scholar]
- Laffón A., Alcocer-Varela J., Alarcón-Segovia D. The autologous mixed lymphocyte reaction is not primarily due to xenoantigenic stimulation. Clin Immunol Immunopathol. 1983 Aug;28(2):304–308. doi: 10.1016/0090-1229(83)90164-2. [DOI] [PubMed] [Google Scholar]
- Lattime E. C., Gillis S., David C., Stutman O. Interleukin 2, production in the syngeneic mixed lymphocyte reaction. Eur J Immunol. 1981 Jan;11(1):67–69. doi: 10.1002/eji.1830110115. [DOI] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Fong S., Dinarello C. A., Carson D. A., Vaughan J. H. Release of lymphokines after infection with Epstein Barr virus in vitro. II. A monocyte-dependent inhibitor of interleukin 1 downregulates the production of interleukin 2 and interferon-gamma in rheumatoid arthritis. J Immunol. 1986 May 15;136(10):3643–3648. [PubMed] [Google Scholar]
- Miossec P., Kashiwado T., Ziff M. Inhibitor of interleukin-2 in rheumatoid synovial fluid. Arthritis Rheum. 1987 Feb;30(2):121–129. doi: 10.1002/art.1780300201. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Romain P. L., Fox D. A., Anderson P., DiMaggio M., Levine H., Schlossman S. F. Abnormalities in CD4+ T-lymphocyte subsets in inflammatory rheumatic diseases. Am J Med. 1988 May;84(5):817–825. doi: 10.1016/0002-9343(88)90058-7. [DOI] [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Möller G. HLA-DR antigens render resting T cells sensitive to interleukin-2 and induce production of the growth factor in the autologous mixed lymphocyte reaction. Cell Immunol. 1981 Sep 1;63(1):143–153. doi: 10.1016/0008-8749(81)90035-6. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Mills M. M. Second-line drug treatment in rheumatoid arthritis associated with depressed autologous mixed lymphocyte reaction. Rheumatol Int. 1986;6(1):25–29. doi: 10.1007/BF00270661. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Murphy J., Panayi G. Abnormal distribution of the helper-inducer and suppressor-inducer T-lymphocyte subsets in the rheumatoid joint. Clin Immunol Immunopathol. 1987 Nov;45(2):252–258. doi: 10.1016/0090-1229(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Pope R. M., McChesney L., Talal N., Fischbach M. Characterization of the defective autologous mixed lymphocyte response in rheumatoid arthritis. Arthritis Rheum. 1984 Nov;27(11):1234–1244. doi: 10.1002/art.1780271105. [DOI] [PubMed] [Google Scholar]
- Romain P. L., Morimoto C., Daley J. F., Palley L. S., Reinherz E. L., Schlossman S. F. Reactivity of inducer cell subsets and T8-cell activation during the human autologous mixed lymphocyte reaction. Clin Immunol Immunopathol. 1984 Jan;30(1):117–128. doi: 10.1016/0090-1229(84)90012-6. [DOI] [PubMed] [Google Scholar]
- Smith J. B., DeHoratius R. J. Deficient autologous mixed lymphocyte reactions correlate with disease activity in systemic lupus erythematosus and rheumatoid arthritis. Clin Exp Immunol. 1982 Apr;48(1):155–162. [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Knowlton R. P. Activation of suppressor T cells in human autologous mixed lymphocyte culture. J Immunol. 1979 Jul;123(1):419–422. [PubMed] [Google Scholar]
- Smolen J. S., Luger T. A., Chused T. M., Steinberg A. D. Responder cells in the human autologous mixed lymphocyte reaction. J Clin Invest. 1981 Dec;68(6):1601–1604. doi: 10.1172/JCI110416. [DOI] [PMC free article] [PubMed] [Google Scholar]


